当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective catalytic asymmetric N-alkylation of isoxazol-5-ones with para-quinone methides
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020/03/14 , DOI: 10.1039/d0ob00393j Suo-Suo Qi 1, 2, 3, 4, 5 , Zhen-Hui Jiang 1, 2, 3, 4, 5 , Ming-Ming Chu 1, 2, 3, 4, 5 , Yi-Feng Wang 1, 2, 3, 4, 5 , Xue-Yang Chen 1, 2, 3, 4, 5 , Wan-Zhen Ju 1, 2, 3, 4, 5 , Dan-Qian Xu 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020/03/14 , DOI: 10.1039/d0ob00393j Suo-Suo Qi 1, 2, 3, 4, 5 , Zhen-Hui Jiang 1, 2, 3, 4, 5 , Ming-Ming Chu 1, 2, 3, 4, 5 , Yi-Feng Wang 1, 2, 3, 4, 5 , Xue-Yang Chen 1, 2, 3, 4, 5 , Wan-Zhen Ju 1, 2, 3, 4, 5 , Dan-Qian Xu 1, 2, 3, 4, 5
Affiliation
|
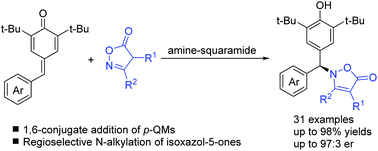
A highly regioselective and enantioselective N-alkylation of isoxazol-5-ones with para-quinone methides promoted by bi-functional squaramide catalysts was developed. This unexpected asymmetric N-addition of isoxazolinones afforded a series of enantioenriched N-diarylmethane substituted isoxazolinones with high yields and enantioselectivities (up to 97 : 3 er). This reaction not only provides a useful approach for intermolecular chiral C–N bond formation but also demonstrates the immense potential of isoxazol-5-ones as N-nucleophiles in catalytic asymmetric reactions.
中文翻译:

异恶唑-5-酮与对醌甲基的区域选择性催化不对称N-烷基化
发展了由双功能方酰胺催化剂促进的对恶唑-5-酮与对醌甲基化物的高度区域选择性和对映选择性的N-烷基化。异恶唑啉酮的这种意想不到的不对称N-加成,提供了一系列高产率和对映选择性(高达97:3 er)的对映体富集的N-二芳基甲烷取代的异恶唑啉酮。该反应不仅为分子间手性C–N键的形成提供了一种有用的方法,而且证明了异恶唑-5-酮作为N-亲核试剂在催化不对称反应中的巨大潜力。
更新日期:2020-04-01
中文翻译:

异恶唑-5-酮与对醌甲基的区域选择性催化不对称N-烷基化
发展了由双功能方酰胺催化剂促进的对恶唑-5-酮与对醌甲基化物的高度区域选择性和对映选择性的N-烷基化。异恶唑啉酮的这种意想不到的不对称N-加成,提供了一系列高产率和对映选择性(高达97:3 er)的对映体富集的N-二芳基甲烷取代的异恶唑啉酮。该反应不仅为分子间手性C–N键的形成提供了一种有用的方法,而且证明了异恶唑-5-酮作为N-亲核试剂在催化不对称反应中的巨大潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号