当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical interaction and enhanced interfacial ion transport in a ceramic nanofiber–polymer composite electrolyte for all-solid-state lithium metal batteries
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020/03/14 , DOI: 10.1039/c9ta12495k Hui Yang 1, 2, 3, 4 , Joeseph Bright 1, 2, 3, 4 , Banghao Chen 4, 5, 6, 7 , Peng Zheng 1, 2, 3, 4 , Xuefei Gao 1, 2, 3, 4 , Botong Liu 1, 2, 3, 4 , Sujan Kasani 1, 2, 3, 4 , Xiangwu Zhang 8, 9, 10, 11, 12 , Nianqiang Wu 1, 2, 3, 4, 13
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020/03/14 , DOI: 10.1039/c9ta12495k Hui Yang 1, 2, 3, 4 , Joeseph Bright 1, 2, 3, 4 , Banghao Chen 4, 5, 6, 7 , Peng Zheng 1, 2, 3, 4 , Xuefei Gao 1, 2, 3, 4 , Botong Liu 1, 2, 3, 4 , Sujan Kasani 1, 2, 3, 4 , Xiangwu Zhang 8, 9, 10, 11, 12 , Nianqiang Wu 1, 2, 3, 4, 13
Affiliation
|
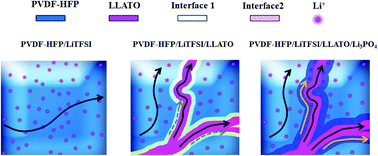
This paper reports the synergy between ceramic nanofibers and a polymer, and the enhanced interfacial Li-ion transport along the nanofiber/polymer interface in a solid-state ceramic/polymer composite electrolyte, in which a three-dimensional (3D) electrospun aluminum-doped Li0.33La0.557TiO3 (LLTO) nanofiber network is embedded in a polyvinylidene fluoride-hexafluoropropylene (PVDF-HFP) matrix. Strong chemical interaction occurs between the nanofibers and the polymer matrix. Addition of the ceramic nanofibers into the polymer matrix results in the dehydrofluorination of the PVDF chains, deprotonation of the –CH2 moiety and amorphization of the polymer matrix. Solid-state nuclear magnetic resonance (NMR) spectra reveal that lithium ions transport via three pathways: (i) intra-polymer transport, (ii) intra-nanofiber transport, and (iii) interfacial polymer/nanofiber transport. In addition, lithium phosphate is coated on the LLTO nanofiber surface before the nanofibers are embedded into the polymer matrix. The presence of lithium phosphate at the LLTO/polymer interface further enhances the chemical interaction between the nanofibers and the polymer, which promotes the lithium ion transport along the polymer/nanofiber interface. This in turn improves the ionic conductivity and electrochemical cycling stability of the nanofiber/polymer composite. As a result, the flexible LLTO/Li3PO4/polymer composite electrolyte membrane exhibits an ionic conductivity of 5.1 × 10−4 S cm−1 at room temperature and an electrochemical stability window of 5.0 V vs. Li/Li+. A symmetric Li|electrolyte|Li half-cell shows a low overpotential of 50 mV at a constant current density of 0.5 mA cm−2 for more than 800 h. In addition, a full cell is constructed by sandwiching the composite electrolyte between a lithium metal anode and a LiFePO4-based cathode. Such an all-solid-state lithium metal battery exhibits excellent cycling performance and rate capability.
中文翻译:

用于全固态锂金属电池的陶瓷纳米纤维-聚合物复合电解质中的化学相互作用和增强的界面离子传输
本文报道了陶瓷纳米纤维与聚合物之间的协同作用,以及在固态陶瓷/聚合物复合电解质中沿纳米纤维/聚合物界面增强的界面锂离子传输,其中三维(3D)电纺铝掺杂Li 0.33 La 0.557 TiO 3(LLTO)纳米纤维网络嵌入聚偏二氟乙烯-六氟丙烯(PVDF-HFP)基质中。纳米纤维和聚合物基质之间发生强烈的化学相互作用。将陶瓷纳米纤维添加到聚合物基体中会导致PVDF链脱氟化氢,–CH 2脱质子聚合物基质的部分和无定形。固态核磁共振(NMR)光谱显示锂离子通过三种途径运输:(i)聚合物内运输,(ii)纳米纤维内运输,和(iii)界面聚合物/纳米纤维运输。另外,在将纳米纤维嵌入聚合物基质之前,将磷酸锂涂覆在LLTO纳米纤维表面上。LLTO /聚合物界面处磷酸锂的存在进一步增强了纳米纤维与聚合物之间的化学相互作用,从而促进了锂离子沿聚合物/纳米纤维界面的传输。这进而改善了纳米纤维/聚合物复合材料的离子电导率和电化学循环稳定性。因此,灵活的LLTO / Li 3 PO 4锂/聚合物复合电解质膜在室温下的离子电导率为5.1×10 -4 S cm -1,相对于Li / Li +的电化学稳定性窗口为5.0V 。对称的Li半电池在800 mA以上的恒定电流密度为0.5 mA cm -2时表现出50 mV的低过电势。另外,通过将复合电解质夹在锂金属阳极和基于LiFePO 4的阴极之间来构造全电池。这种全固态锂金属电池表现出优异的循环性能和倍率性能。
更新日期:2020-04-15
中文翻译:

用于全固态锂金属电池的陶瓷纳米纤维-聚合物复合电解质中的化学相互作用和增强的界面离子传输
本文报道了陶瓷纳米纤维与聚合物之间的协同作用,以及在固态陶瓷/聚合物复合电解质中沿纳米纤维/聚合物界面增强的界面锂离子传输,其中三维(3D)电纺铝掺杂Li 0.33 La 0.557 TiO 3(LLTO)纳米纤维网络嵌入聚偏二氟乙烯-六氟丙烯(PVDF-HFP)基质中。纳米纤维和聚合物基质之间发生强烈的化学相互作用。将陶瓷纳米纤维添加到聚合物基体中会导致PVDF链脱氟化氢,–CH 2脱质子聚合物基质的部分和无定形。固态核磁共振(NMR)光谱显示锂离子通过三种途径运输:(i)聚合物内运输,(ii)纳米纤维内运输,和(iii)界面聚合物/纳米纤维运输。另外,在将纳米纤维嵌入聚合物基质之前,将磷酸锂涂覆在LLTO纳米纤维表面上。LLTO /聚合物界面处磷酸锂的存在进一步增强了纳米纤维与聚合物之间的化学相互作用,从而促进了锂离子沿聚合物/纳米纤维界面的传输。这进而改善了纳米纤维/聚合物复合材料的离子电导率和电化学循环稳定性。因此,灵活的LLTO / Li 3 PO 4锂/聚合物复合电解质膜在室温下的离子电导率为5.1×10 -4 S cm -1,相对于Li / Li +的电化学稳定性窗口为5.0V 。对称的Li半电池在800 mA以上的恒定电流密度为0.5 mA cm -2时表现出50 mV的低过电势。另外,通过将复合电解质夹在锂金属阳极和基于LiFePO 4的阴极之间来构造全电池。这种全固态锂金属电池表现出优异的循环性能和倍率性能。










































 京公网安备 11010802027423号
京公网安备 11010802027423号