当前位置:
X-MOL 学术
›
Signal Transduct. Target Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combined inhibition of Notch and FLT3 produces synergistic cytotoxic effects in FLT3/ITD+ acute myeloid leukemia.
Signal Transduction and Targeted Therapy ( IF 40.8 ) Pub Date : 2020-03-13 , DOI: 10.1038/s41392-020-0108-z Dan Li 1 , Tongjuan Li 1 , Zhen Shang 1 , Lei Zhao 1 , Qian Xu 1, 2 , Jiaqi Tan 1 , Yun Qin 1 , Yuanyuan Zhang 1 , Yang Cao 1 , Na Wang 1, 3 , Liang Huang 1 , Xiaojian Zhu 1 , Kuangguo Zhou 1 , Liting Chen 1, 3 , Chunrui Li 1 , Ting Xie 4 , Yi Yang 5 , Jue Wang 1 , Jianfeng Zhou 1
Signal Transduction and Targeted Therapy ( IF 40.8 ) Pub Date : 2020-03-13 , DOI: 10.1038/s41392-020-0108-z Dan Li 1 , Tongjuan Li 1 , Zhen Shang 1 , Lei Zhao 1 , Qian Xu 1, 2 , Jiaqi Tan 1 , Yun Qin 1 , Yuanyuan Zhang 1 , Yang Cao 1 , Na Wang 1, 3 , Liang Huang 1 , Xiaojian Zhu 1 , Kuangguo Zhou 1 , Liting Chen 1, 3 , Chunrui Li 1 , Ting Xie 4 , Yi Yang 5 , Jue Wang 1 , Jianfeng Zhou 1
Affiliation
|
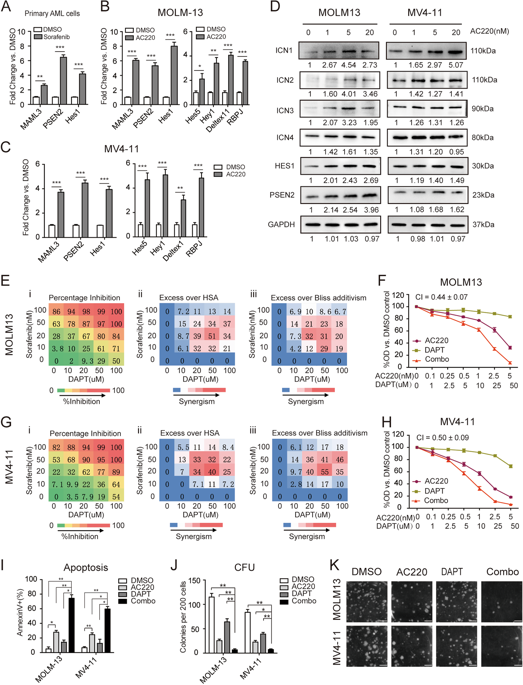
Internal tandem duplication (ITD) mutations of FMS-like tyrosine kinase-3 (FLT3) are the most frequent genetic alterations in acute myeloid leukemia (AML) and predict a poor prognosis. FLT3 tyrosine kinase inhibitors (TKIs) provide short-term clinical responses, but the long-term prognosis of FLT3/ITD+ AML patients remains poor. Notch signaling is important in numerous types of tumors. However, the role of Notch signaling in FLT3/ITD+ AML remains to be elucidated. In the current study, we found that Notch signaling was activated upon FLT3-TKI treatment in FLT3/ITD+ cell lines and primary cells. As Notch signaling can be blocked by γ-secretase inhibitors (GSIs), we examined the combinatorial antitumor efficacy of FLT3-TKIs and GSIs against FLT3/ITD+ AML and explored the underlying molecular mechanisms. As a result, we observed synergistic cytotoxic effects, and the treatment preferentially reduced cell proliferation and induced apoptosis in FLT3/ITD+ AML cell lines and in primary AML cells. Furthermore, the combination of FLT3-TKI and GSI eradicated leukemic cells and prolonged survival in an FLT3/ITD+ patient-derived xenograft AML model. Mechanistically, differential expression analysis suggested that CXCR3 may be partially responsible for the observed synergy, possibly through ERK signaling. Our findings suggest that combined therapies of FLT3-TKIs with GSI may be exploited as a potential therapeutic strategy to treat FLT3/ITD+ AML.
中文翻译:

Notch 和 FLT3 的联合抑制在 FLT3/ITD+ 急性髓系白血病中产生协同细胞毒作用。
FMS 样酪氨酸激酶-3 (FLT3) 的内部串联重复 (ITD) 突变是急性髓系白血病 (AML) 中最常见的遗传改变,并预示着预后不良。FLT3 酪氨酸激酶抑制剂 (TKI) 提供短期临床反应,但 FLT3/ITD+ AML 患者的长期预后仍然很差。Notch 信号在许多类型的肿瘤中都很重要。然而,Notch 信号在 FLT3/ITD+ AML 中的作用仍有待阐明。在目前的研究中,我们发现在 FLT3/ITD+ 细胞系和原代细胞中 FLT3-TKI 处理后 Notch 信号被激活。由于 Notch 信号传导可以被 γ-分泌酶抑制剂 (GSI) 阻断,我们检查了 FLT3-TKI 和 GSI 对 FLT3/ITD+ AML 的组合抗肿瘤功效,并探索了潜在的分子机制。其结果,我们观察到协同细胞毒作用,并且治疗优先减少 FLT3/ITD+ AML 细胞系和原代 AML 细胞中的细胞增殖并诱导细胞凋亡。此外,FLT3-TKI 和 GSI 的组合在 FLT3/ITD+ 患者来源的异种移植 AML 模型中根除白血病细胞并延长了存活时间。从机制上讲,差异表达分析表明 CXCR3 可能是观察到的协同作用的部分原因,可能是通过 ERK 信号传导。我们的研究结果表明,FLT3-TKI 与 GSI 的联合治疗可作为治疗 FLT3/ITD+ AML 的潜在治疗策略。FLT3-TKI 和 GSI 的组合在 FLT3/ITD+ 患者来源的异种移植 AML 模型中根除白血病细胞并延长了存活时间。从机制上讲,差异表达分析表明 CXCR3 可能是观察到的协同作用的部分原因,可能是通过 ERK 信号传导。我们的研究结果表明,FLT3-TKI 与 GSI 的联合治疗可作为治疗 FLT3/ITD+ AML 的潜在治疗策略。FLT3-TKI 和 GSI 的组合在 FLT3/ITD+ 患者来源的异种移植 AML 模型中根除白血病细胞并延长了存活时间。从机制上讲,差异表达分析表明 CXCR3 可能是观察到的协同作用的部分原因,可能是通过 ERK 信号传导。我们的研究结果表明,FLT3-TKI 与 GSI 的联合治疗可作为治疗 FLT3/ITD+ AML 的潜在治疗策略。
更新日期:2020-03-13
中文翻译:

Notch 和 FLT3 的联合抑制在 FLT3/ITD+ 急性髓系白血病中产生协同细胞毒作用。
FMS 样酪氨酸激酶-3 (FLT3) 的内部串联重复 (ITD) 突变是急性髓系白血病 (AML) 中最常见的遗传改变,并预示着预后不良。FLT3 酪氨酸激酶抑制剂 (TKI) 提供短期临床反应,但 FLT3/ITD+ AML 患者的长期预后仍然很差。Notch 信号在许多类型的肿瘤中都很重要。然而,Notch 信号在 FLT3/ITD+ AML 中的作用仍有待阐明。在目前的研究中,我们发现在 FLT3/ITD+ 细胞系和原代细胞中 FLT3-TKI 处理后 Notch 信号被激活。由于 Notch 信号传导可以被 γ-分泌酶抑制剂 (GSI) 阻断,我们检查了 FLT3-TKI 和 GSI 对 FLT3/ITD+ AML 的组合抗肿瘤功效,并探索了潜在的分子机制。其结果,我们观察到协同细胞毒作用,并且治疗优先减少 FLT3/ITD+ AML 细胞系和原代 AML 细胞中的细胞增殖并诱导细胞凋亡。此外,FLT3-TKI 和 GSI 的组合在 FLT3/ITD+ 患者来源的异种移植 AML 模型中根除白血病细胞并延长了存活时间。从机制上讲,差异表达分析表明 CXCR3 可能是观察到的协同作用的部分原因,可能是通过 ERK 信号传导。我们的研究结果表明,FLT3-TKI 与 GSI 的联合治疗可作为治疗 FLT3/ITD+ AML 的潜在治疗策略。FLT3-TKI 和 GSI 的组合在 FLT3/ITD+ 患者来源的异种移植 AML 模型中根除白血病细胞并延长了存活时间。从机制上讲,差异表达分析表明 CXCR3 可能是观察到的协同作用的部分原因,可能是通过 ERK 信号传导。我们的研究结果表明,FLT3-TKI 与 GSI 的联合治疗可作为治疗 FLT3/ITD+ AML 的潜在治疗策略。FLT3-TKI 和 GSI 的组合在 FLT3/ITD+ 患者来源的异种移植 AML 模型中根除白血病细胞并延长了存活时间。从机制上讲,差异表达分析表明 CXCR3 可能是观察到的协同作用的部分原因,可能是通过 ERK 信号传导。我们的研究结果表明,FLT3-TKI 与 GSI 的联合治疗可作为治疗 FLT3/ITD+ AML 的潜在治疗策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号