Prostate Cancer and Prostatic Diseases ( IF 5.1 ) Pub Date : 2020-03-13 , DOI: 10.1038/s41391-020-0221-7 Joseph Longo 1, 2 , Robert J Hamilton 1, 3 , Mehdi Masoomian 4 , Najia Khurram 1, 3 , Emily Branchard 1 , Peter J Mullen 1 , Mohamad Elbaz 1 , Karen Hersey 1, 3 , Dianne Chadwick 4 , Sangeet Ghai 1, 5 , David W Andrews 2, 6 , Eric X Chen 1 , Theodorus H van der Kwast 4 , Neil E Fleshner 1, 3 , Linda Z Penn 1, 2
|
Background
Statins inhibit HMG-CoA reductase, the rate-limiting enzyme of the mevalonate pathway. Epidemiological and pre-clinical evidence support an association between statin use and delayed prostate cancer (PCa) progression. Here, we evaluated the effects of neoadjuvant fluvastatin treatment on markers of cell proliferation and apoptosis in men with localized PCa.
Methods
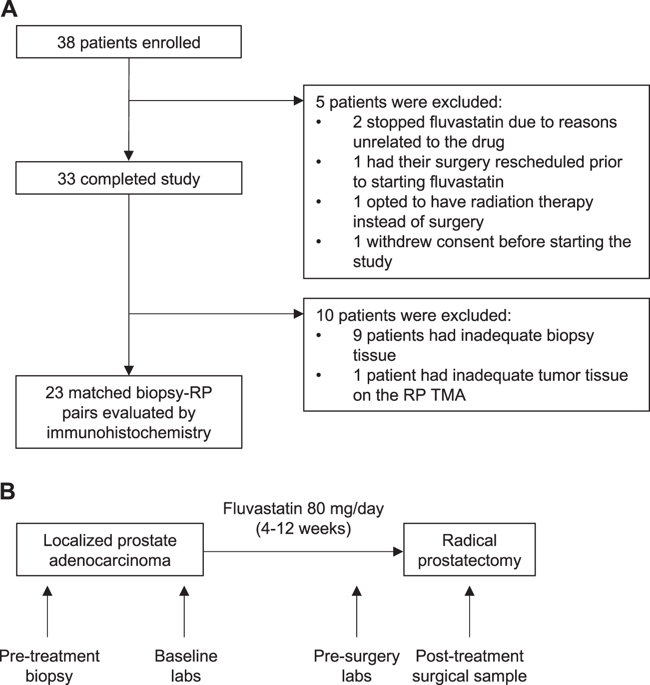
Thirty-three men were treated daily with 80 mg fluvastatin for 4–12 weeks in a single-arm window-of-opportunity study between diagnosis of localized PCa and radical prostatectomy (RP) (ClinicalTrials.gov: NCT01992042). Percent Ki67 and cleaved Caspase-3 (CC3)-positive cells in tumor tissues were evaluated in 23 patients by immunohistochemistry before and after treatment. Serum and intraprostatic fluvastatin concentrations were quantified by liquid chromatography-mass spectrometry.
Results
Baseline characteristics included a median prostate-specific antigen (PSA) level of 6.48 ng/mL (IQR: 4.21–10.33). The median duration of fluvastatin treatment was 49 days (range: 27–102). Median serum low-density lipoprotein levels decreased by 35% after treatment, indicating patient compliance. Median PSA decreased by 12%, but this was not statistically significant in our small cohort. The mean fluvastatin concentration measured in the serum was 0.2 μM (range: 0.0–1.1 μM), and in prostatic tissue was 8.5 nM (range: 0.0–77.0 nM). At these concentrations, fluvastatin induced PCa cell death in vitro in a dose- and time-dependent manner. In patients, fluvastatin treatment did not significantly alter intratumoral Ki67 positivity; however, a median 2.7-fold increase in CC3 positivity (95% CI: 1.9–5.0, p = 0.007) was observed in post-fluvastatin RP tissues compared with matched pre-treatment biopsy controls. In a subset analysis, this increase in CC3 was more pronounced in men on fluvastatin for >50 days.
Conclusions
Fluvastatin prior to RP achieves measurable drug concentrations in prostatic tissue and is associated with promising effects on tumor cell apoptosis. These data warrant further investigation into the anti-neoplastic effects of statins in prostate tissue.
中文翻译:

局部前列腺癌术前氟伐他汀的初步机会窗研究
背景
他汀类药物抑制 HMG-CoA 还原酶,甲羟戊酸途径的限速酶。流行病学和临床前证据支持他汀类药物的使用与前列腺癌 (PCa) 进展延迟之间存在关联。在这里,我们评估了新辅助氟伐他汀治疗对局部 PCa 男性细胞增殖和凋亡标志物的影响。
方法
在局部 PCa 诊断和根治性前列腺切除术 (RP) 之间的单臂机会窗研究中,33 名男性每天接受 80 毫克氟伐他汀治疗,持续 4-12 周(ClinicalTrials.gov:NCT01992042)。在治疗前后通过免疫组织化学评估了 23 名患者的肿瘤组织中 Ki67 和裂解的 Caspase-3 (CC3) 阳性细胞的百分比。血清和前列腺内氟伐他汀浓度通过液相色谱-质谱法定量。
结果
基线特征包括中位前列腺特异性抗原 (PSA) 水平为 6.48 ng/mL(IQR:4.21–10.33)。氟伐他汀治疗的中位持续时间为 49 天(范围:27-102)。治疗后中位血清低密度脂蛋白水平下降了 35%,表明患者依从性。中位 PSA 下降了 12%,但这在我们的小队列中没有统计学意义。血清中测得的平均氟伐他汀浓度为 0.2 μM(范围:0.0–1.1 μM),前列腺组织中测得的平均氟伐他汀浓度为 8.5 nM(范围:0.0–77.0 nM)。在这些浓度下,氟伐他汀在体外以剂量和时间依赖性方式诱导 PCa 细胞死亡。在患者中,氟伐他汀治疗没有显着改变瘤内 Ki67 阳性;然而,CC3 阳性率中位数增加了 2.7 倍(95% CI:1.9-5.0,p = 0.007) 与匹配的治疗前活检对照相比,在氟伐他汀后 RP 组织中观察到。在子集分析中,CC3 的这种增加在服用氟伐他汀超过 50 天的男性中更为明显。
结论
在 RP 之前使用氟伐他汀在前列腺组织中达到可测量的药物浓度,并与对肿瘤细胞凋亡的有希望的影响有关。这些数据值得进一步研究他汀类药物在前列腺组织中的抗肿瘤作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号