Cell Death & Disease ( IF 8.1 ) Pub Date : 2020-03-13 , DOI: 10.1038/s41419-020-2365-8 Ji-Hyeon Lee 1 , Jose Manuel Mellado-Gil 1, 2 , Young Jae Bahn 1 , Sushrut M Pathy 1 , Ying E Zhang 3 , Sushil G Rane 1
|
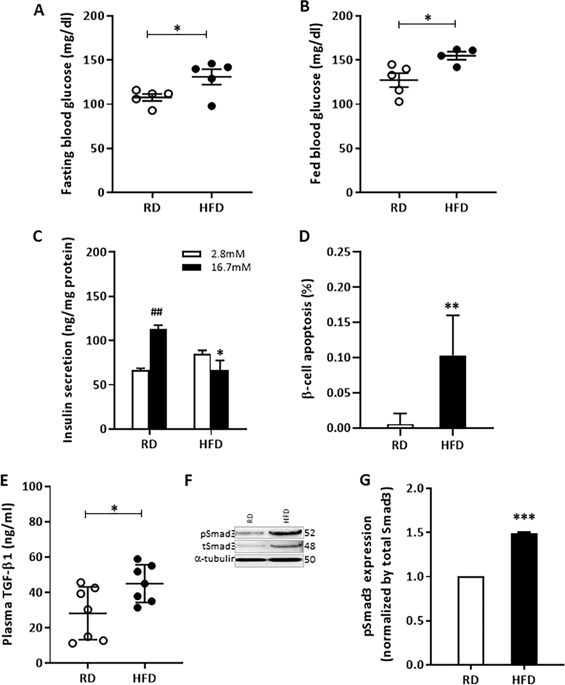
Prevailing insulin resistance and the resultant hyperglycemia elicits a compensatory response from pancreatic islet beta cells (β-cells) that involves increases in β-cell function and β-cell mass. However, the sustained metabolic stress eventually leads to β-cell failure characterized by severe β-cell dysfunction and progressive loss of β-cell mass. Whereas, β-cell dysfunction is relatively well understood at the mechanistic level, the avenues leading to loss of β-cell mass are less clear with reduced proliferation, dedifferentiation, and apoptosis all potential mechanisms. Butler and colleagues documented increased β-cell apoptosis in pancreas from lean and obese human Type 2 diabetes (T2D) subjects, with no changes in rates of β-cell replication or neogenesis, strongly suggesting a role for apoptosis in β-cell failure. Here, we describe a permissive role for TGF-β/Smad3 in β-cell apoptosis. Human islets undergoing β-cell apoptosis release increased levels of TGF-β1 ligand and phosphorylation levels of TGF-β’s chief transcription factor, Smad3, are increased in human T2D islets suggestive of an autocrine role for TGF-β/Smad3 signaling in β-cell apoptosis. Smad3 phosphorylation is similarly increased in diabetic mouse islets undergoing β-cell apoptosis. In mice, β-cell-specific activation of Smad3 promotes apoptosis and loss of β-cell mass in association with β-cell dysfunction, glucose intolerance, and diabetes. In contrast, inactive Smad3 protects from apoptosis and preserves β-cell mass while improving β-cell function and glucose tolerance. At the molecular level, Smad3 associates with Foxo1 to propagate TGF-β-dependent β-cell apoptosis. Indeed, genetic or pharmacologic inhibition of TGF-β/Smad3 signals or knocking down Foxo1 protects from β-cell apoptosis. These findings reveal the importance of TGF-β/Smad3 in promoting β-cell apoptosis and demonstrate the therapeutic potential of TGF-β/Smad3 antagonism to restore β-cell mass lost in diabetes.
中文翻译:

通过抑制 TGF-β/Smad3 信号转导防止 β 细胞凋亡
普遍的胰岛素抵抗和由此产生的高血糖引起胰岛 β 细胞(β 细胞)的代偿反应,包括 β 细胞功能和 β 细胞质量的增加。然而,持续的代谢应激最终导致 β 细胞衰竭,其特征是严重的 β 细胞功能障碍和 β 细胞质量的进行性损失。然而,β 细胞功能障碍在机制水平上相对较好理解,导致 β 细胞质量损失的途径不太清楚,增殖减少、去分化和细胞凋亡是所有潜在机制。Butler 及其同事记录了消瘦和肥胖的人类 2 型糖尿病 (T2D) 受试者的胰腺中 β 细胞凋亡增加,而 β 细胞复制或新生率没有变化,强烈表明细胞凋亡在 β 细胞衰竭中起作用。这里,我们描述了 TGF-β/Smad3 在 β 细胞凋亡中的许可作用。经历 β 细胞凋亡的人胰岛释放出更高水平的 TGF-β1 配体,并且 TGF-β 的主要转录因子 Smad3 的磷酸化水平在人 T2D 胰岛中增加,表明 TGF-β/Smad3 信号在 β 细胞中的自分泌作用细胞凋亡。在经历 β 细胞凋亡的糖尿病小鼠胰岛中,Smad3 磷酸化同样增加。在小鼠中,Smad3 的 β 细胞特异性激活会促进与 β 细胞功能障碍、葡萄糖耐受不良和糖尿病相关的细胞凋亡和 β 细胞质量损失。相反,无活性的 Smad3 可防止细胞凋亡并保持 β 细胞质量,同时改善 β 细胞功能和葡萄糖耐量。在分子水平上,Smad3 与 Foxo1 结合以传播 TGF-β 依赖性 β 细胞凋亡。的确,TGF-β/Smad3 信号的遗传或药物抑制或敲低 Foxo1 可防止 β 细胞凋亡。这些发现揭示了 TGF-β/Smad3 在促进 β 细胞凋亡中的重要性,并证明了 TGF-β/Smad3 拮抗作用在恢复糖尿病中损失的 β 细胞量方面的治疗潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号