The Lancet Respiratory Medicine ( IF 38.7 ) Pub Date : 2020-03-13 , DOI: 10.1016/s2213-2600(19)30469-2 Carolin T Turner 1 , Rishi K Gupta 2 , Evdokia Tsaliki 1 , Jennifer K Roe 1 , Prasenjit Mondal 1 , Georgina R Nyawo 3 , Zaida Palmer 3 , Robert F Miller 2 , Byron Wp Reeve 3 , Grant Theron 3 , Mahdad Noursadeghi 1
|
Background
Blood transcriptional signatures are candidates for non-sputum triage or confirmatory tests of tuberculosis. Prospective head-to-head comparisons of their diagnostic accuracy in real-world settings are necessary to assess their clinical use. We aimed to compare the diagnostic accuracy of candidate transcriptional signatures identified by systematic review, in a setting with a high burden of tuberculosis and HIV.
Methods
We did a prospective observational study nested within a diagnostic accuracy study of sputum Xpert MTB/RIF (Xpert) and Xpert MTB/RIF Ultra (Ultra) tests for pulmonary tuberculosis. We recruited consecutive symptomatic adults aged 18 years or older self-presenting to a tuberculosis clinic in Cape Town, South Africa. Participants provided blood for RNA sequencing, and sputum samples for liquid culture and molecular testing using Xpert and Ultra. We assessed the diagnostic accuracy of candidate blood transcriptional signatures for active tuberculosis (including those intended to distinguish active tuberculosis from other diseases) identified by systematic review, compared with culture or Xpert MTB/RIF positivity as the standard reference. In our primary analysis, patients with tuberculosis were defined as those with either a positive liquid culture or Xpert result. Patients with missing blood RNA or sputum results were excluded. Our primary objective was to benchmark the diagnostic accuracy of candidate transcriptional signatures against the WHO target product profile (TPP) for a tuberculosis triage test.
Findings
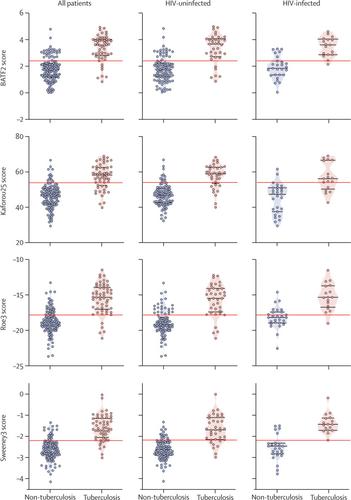
Between Feb 12, 2016, and July 18, 2017, we obtained paired sputum and RNA sequencing data from 181 participants, 54 (30%) of whom had confirmed pulmonary tuberculosis. Of 27 eligible signatures identified by systematic review, four achieved the highest diagnostic accuracy with similar area under the receiver operating characteristic curves (Sweeney3: 90·6% [95% CI 85·6–95·6]; Kaforou25: 86·9% [80·9–92·9]; Roe3: 86·9% [80·3–93·5]; and BATF2: 86·8% [80·6–93·1]), independent of age, sex, HIV status, previous tuberculosis, or sputum smear result. At test thresholds that gave 70% specificity (the minimum WHO TPP specificity for a triage test), these four signatures achieved sensitivities between 83·3% (95% CI 71·3–91·0) and 90·7% (80·1–96·0). No signature met the optimum criteria, of 95% sensitivity and 80% specificity proposed by WHO for a triage test, or the minimum criteria (of 65% sensitivity and 98% specificity) for a confirmatory test, but all four correctly identified Ultra-positive, culture-negative patients.
Interpretation
Selected blood transcriptional signatures met the minimum WHO benchmarks for a tuberculosis triage test but not for a confirmatory test. Further development of the signatures is warranted to investigate their possible effects on clinical and health economic outcomes as part of a triage strategy, or when used as add-on confirmatory test in conjunction with the highly sensitive Ultra test for Mycobacterium tuberculosis DNA.
Funding
Royal Society Newton Advanced Fellowship, Wellcome Trust, National Institute of Health Research, and UK Medical Research Council.
中文翻译:

高负担环境中活动性肺结核的血液转录生物标志物:一项前瞻性、观察性、诊断准确性研究。
背景
血液转录特征是结核病非痰分类或确认测试的候选者。为了评估其临床应用,有必要在现实环境中对其诊断准确性进行前瞻性的头对头比较。我们的目的是在结核病和艾滋病毒高负担的环境中比较通过系统评价确定的候选转录特征的诊断准确性。
方法
我们进行了一项前瞻性观察研究,该研究包含在痰液 Xpert MTB/RIF (Xpert) 和 Xpert MTB/RIF Ultra (Ultra) 肺结核检测诊断准确性研究中。我们连续招募了 18 岁或以上有症状且自行到南非开普敦一家结核病诊所就诊的成年人。参与者提供血液用于 RNA 测序,并提供痰样本使用 Xpert 和 Ultra 进行液体培养和分子检测。我们评估了通过系统评价确定的活动性结核病候选血液转录特征(包括旨在区分活动性结核病与其他疾病的特征)的诊断准确性,并与培养物或 Xpert MTB/RIF 阳性作为标准参考进行比较。在我们的初步分析中,结核病患者被定义为液体培养或 Xpert 结果呈阳性的患者。血液 RNA 或痰液结果缺失的患者被排除在外。我们的主要目标是根据结核病分类测试的 WHO 目标产品概况 (TPP) 对候选转录特征的诊断准确性进行基准测试。
发现
2016年2月12日至2017年7月18日期间,我们获得了181名参与者的配对痰液和RNA测序数据,其中54人(30%)已确诊肺结核。在系统审查确定的 27 个合格特征中,有 4 个特征达到了最高的诊断准确度,且受试者工作特征曲线下的面积相似(Sweeney3:90·6% [95% CI 85·6–95·6];Kaforou25:86·9%) [80·9–92·9];Roe3:86·9% [80·3–93·5];BATF2:86·8% [80·6–93·1]),与年龄、性别无关HIV 状况、既往结核病史或痰涂片结果。在给出 70% 特异性的测试阈值(分类测试的最低 WHO TPP 特异性)下,这四个特征的灵敏度在 83·3% (95% CI 71·3–91·0) 和 90·7% (80· 1–96·0)。没有签名符合世界卫生组织提出的分类测试的最佳标准,即 95% 的敏感性和 80% 的特异性,或确认测试的最低标准(65% 的敏感性和 98% 的特异性),但所有四个都正确识别为 超阳性,培养阴性患者。
解释
选定的血液转录特征符合结核病分类测试的最低世界卫生组织基准,但不符合确认测试的最低标准。作为分诊策略的一部分,或者当与结核分枝杆菌DNA 的高灵敏度 Ultra 测试结合使用时,需要进一步开发这些特征来研究它们对临床和健康经济结果的可能影响。
资金
英国皇家学会牛顿高级研究员、威康信托基金、国家健康研究所和英国医学研究委员会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号