Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial.
The Lancet ( IF 98.4 ) Pub Date : 2020-03-13 , DOI: 10.1016/s0140-6736(20)30263-4 Philip J Mease 1 , Proton Rahman 2 , Alice B Gottlieb 3 , Alexa P Kollmeier 4 , Elizabeth C Hsia 5 , Xie L Xu 4 , Shihong Sheng 6 , Prasheen Agarwal 6 , Bei Zhou 6 , Yanli Zhuang 7 , Désirée van der Heijde 8 , Iain B McInnes 9 ,
中文翻译:

Guselkumab用于初生性活动性银屑病关节炎(DISCOVER-2)的患者:一项双盲,随机,安慰剂对照的3期临床试验。
更新日期:2020-04-03
The Lancet ( IF 98.4 ) Pub Date : 2020-03-13 , DOI: 10.1016/s0140-6736(20)30263-4 Philip J Mease 1 , Proton Rahman 2 , Alice B Gottlieb 3 , Alexa P Kollmeier 4 , Elizabeth C Hsia 5 , Xie L Xu 4 , Shihong Sheng 6 , Prasheen Agarwal 6 , Bei Zhou 6 , Yanli Zhuang 7 , Désirée van der Heijde 8 , Iain B McInnes 9 ,
Affiliation
|
Background
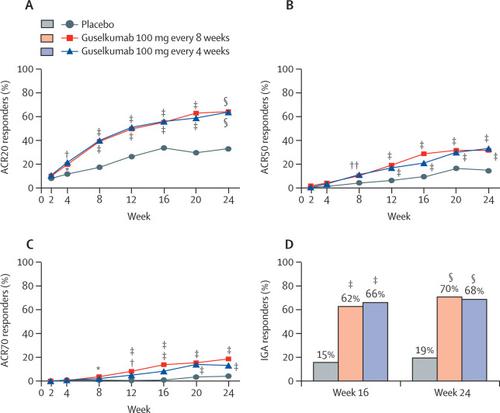
The interleukin-23 (IL-23)/T-helper 17 cell pathway is implicated in psoriatic arthritis pathogenesis. Guselkumab, an IL-23 inhibitor that specifically binds the IL-23 p19 subunit, significantly and safely improved psoriatic arthritis in a phase 2 study. DISCOVER-2 was a phase 3 trial to assess guselkumab in biologic-naive patients with psoriatic arthritis.Methods
This phase 3, double-blind, placebo-controlled study was done at 118 sites in 13 countries across Asia, Europe, and North America. We enrolled biologic-naive patients with active psoriatic arthritis (at least five swollen joints, at least five tender joints, and C-reactive protein ≥0·6 mg/dL) despite standard therapies. Patients were randomly assigned (1:1:1, computer-generated permuted blocks; stratified by baseline disease-modifying antirheumatic drug use and C-reactive protein concentration) to subcutaneous injections of guselkumab 100 mg every 4 weeks; guselkumab 100 mg at weeks 0, 4, then every 8 weeks; or placebo. The primary endpoint was American College of Rheumatology 20% improvement (ACR20) response at week 24 in all patients per assigned treatment group. Safety was assessed in all patients per treatment received. This trial is registered at , (active, not recruiting).Findings
From July 13, 2017, to Aug 3, 2018, 1153 patients were screened, of whom 741 were randomly assigned to receive guselkumab every 4 weeks (n=246), every 8 weeks (n=248), or placebo (n=247). One patient in the every 4 weeks group and one in the placebo group did not start treatment, and the remaining 739 patients started treatment; 716 patients continued treatment up to week 24. Significantly greater proportions of patients in the guselkumab every 4 weeks group (156 [64%] of 245 [95% CI 57–70]) and every 8 weeks group (159 [64%] of 248 [58–70]) than in the placebo group (81 [33%] of 246 [27–39]) achieved an ACR20 response at week 24 (percentage differences vs placebo 31% [95% CI 22–39] for the every 4 weeks group and 31% [23–40] for the every 8 weeks group; both p<0·0001). Up to week 24, serious adverse events occurred in eight (3%) of 245 patients receiving guselkumab every 4 weeks (three serious infections), three (1%) of 248 receiving guselkumab every 8 weeks (one serious infection), and seven (3%) of 246 receiving placebo (one serious infection). No deaths occurred.Interpretation
Guselkumab, a human monoclonal antibody that specifically inhibits IL-23 by binding the cytokine's p19 subunit, was efficacious and demonstrated an acceptable benefit–risk profile in patients with active psoriatic arthritis who were naive to treatment with biologics. These data support the use of selective inhibition of IL-23 to treat psoriatic arthritis.Funding
Janssen Research and Development.中文翻译:

Guselkumab用于初生性活动性银屑病关节炎(DISCOVER-2)的患者:一项双盲,随机,安慰剂对照的3期临床试验。











































 京公网安备 11010802027423号
京公网安备 11010802027423号