当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Room-temperature Pd/Ag direct arylation enabled by a radical pathway
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-03-13 , DOI: 10.3762/bjoc.16.36 Amy L Mayhugh , Christine K Luscombe
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-03-13 , DOI: 10.3762/bjoc.16.36 Amy L Mayhugh , Christine K Luscombe
|
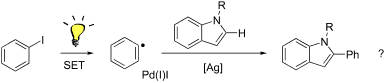
Direct arylation is an appealing method for preparing π-conjugated materials, avoiding the prefunctionalization required for traditional cross-coupling methods. A major effort in organic electronic materials development is improving the environmental and economic impact of production; direct arylation polymerization (DArP) is an effective method to achieve these goals. Room-temperature polymerization would further improve the cost and energy efficiencies required to prepare these materials. Reported herein is new mechanistic work studying the underlying mechanism of room temperature direct arylation between iodobenzene and indole. Results indicate that room-temperature, Pd/Ag-catalyzed direct arylation systems are radical-mediated. This is in contrast to the commonly proposed two-electron mechanisms for direct arylation and appears to extend to other substrates such as benzo[b]thiophene and pentafluorobenzene.
中文翻译:

自由基途径使室温Pd / Ag直接芳基化
直接芳基化是制备π共轭材料的一种有吸引力的方法,避免了传统交叉偶联方法所需的预功能化。有机电子材料开发的一项主要工作是改善生产对环境和经济的影响;直接芳基化聚合(DArP)是实现这些目标的有效方法。室温聚合将进一步改善制备这些材料所需的成本和能量效率。本文报道的是新的机理研究,研究了碘苯与吲哚之间室温直接芳基化的潜在机理。结果表明,室温下Pd / Ag催化的直接芳基化体系是自由基介导的。b ]噻吩和五氟苯。
更新日期:2020-03-16
中文翻译:

自由基途径使室温Pd / Ag直接芳基化
直接芳基化是制备π共轭材料的一种有吸引力的方法,避免了传统交叉偶联方法所需的预功能化。有机电子材料开发的一项主要工作是改善生产对环境和经济的影响;直接芳基化聚合(DArP)是实现这些目标的有效方法。室温聚合将进一步改善制备这些材料所需的成本和能量效率。本文报道的是新的机理研究,研究了碘苯与吲哚之间室温直接芳基化的潜在机理。结果表明,室温下Pd / Ag催化的直接芳基化体系是自由基介导的。b ]噻吩和五氟苯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号