Molecular Therapy - Methods & Clinical Development ( IF 4.6 ) Pub Date : 2020-03-14 , DOI: 10.1016/j.omtm.2020.03.002 Seyedeh Zeinab Mirjalili Mohanna , Jack W. Hickmott , Siu Ling Lam , Nina Y. Chiu , Tess C. Lengyell , Beatrice M. Tam , Orson L. Moritz , Elizabeth M. Simpson
|
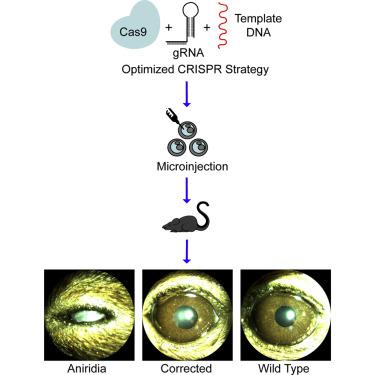
Aniridia is a rare eye disorder, which is caused by mutations in the paired box 6 (PAX6) gene and results in vision loss due to the lack of a long-term vision-saving therapy. One potential approach to treating aniridia is targeted CRISPR-based genome editing. To enable the Pax6 small eye (Sey) mouse model of aniridia, which carries the same mutation found in patients, for preclinical testing of CRISPR-based therapeutic approaches, we endogenously tagged the Sey allele, allowing for the differential detection of protein from each allele. We optimized a correction strategy in vitro then tested it in vivo in the germline of our new mouse to validate the causality of the Sey mutation. The genomic manipulations were analyzed by PCR, as well as by Sanger and next-generation sequencing. The mice were studied by slit lamp imaging, immunohistochemistry, and western blot analyses. We successfully achieved both in vitro and in vivo germline correction of the Sey mutation, with the former resulting in an average 34.8% ± 4.6% SD correction, and the latter in restoration of 3xFLAG-tagged PAX6 expression and normal eyes. Hence, in this study we have created a novel mouse model for aniridia, demonstrated that germline correction of the Sey mutation alone rescues the mutant phenotype, and developed an allele-distinguishing CRISPR-based strategy for aniridia.
中文翻译:

Germline CRISPR / Cas9介导的基因编辑可防止新的无虹膜小鼠模型失明
Aniridia是一种罕见的眼疾,它是由配对box 6(PAX6)基因的突变引起的,由于缺乏长期的节省视力的治疗方法而导致视力丧失。一种治疗虹膜虹膜的潜在方法是基于CRISPR的靶向基因组编辑。为了使携带患者中相同突变的无虹膜Pax6小眼(Sey)小鼠模型能够用于基于CRISPR的治疗方法的临床前测试,我们内源标记了Sey等位基因,从而可以从每个等位基因中进行差异检测。我们在体外优化了一种校正策略,然后在新小鼠的种系中对其进行了体内测试,以验证其成因。Sey突变。通过PCR,Sanger和下一代测序分析了基因组操作。通过裂隙灯成像,免疫组织化学和蛋白质印迹分析研究了小鼠。我们成功地实现了Sey突变的体外和体内种系校正,前者平均可实现34.8%±4.6%SD校正,而后者可恢复3xFLAG标签的PAX6表达和正常眼睛。因此,在这项研究中,我们创建了一种用于无虹膜的新型小鼠模型,证明仅靠Sey突变的种系校正就能拯救突变表型,并开发了基于等位基因的基于CRISPR的无虹膜策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号