当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic properties of variously immobilized mushroom tyrosinase: A kinetic study for future development of biomimetic amperometric biosensors
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jelechem.2020.114066 Milan Sýs , Michaela Obluková , Viliam Kolivoška , Romana Sokolová , Lucie Korecká , Tomáš Mikysek
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jelechem.2020.114066 Milan Sýs , Michaela Obluková , Viliam Kolivoška , Romana Sokolová , Lucie Korecká , Tomáš Mikysek
|
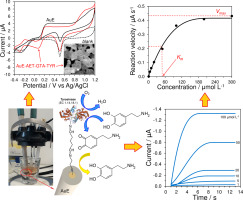
Abstract Mushroom tyrosinase was immobilized by direct embedding into electrode material (modified carbon paste electrode), incorporation of cross-linked enzyme aggregates into a polymer membrane (glassy carbon electrode covered by thin layer of Nafion®), and covalent attachment using self-assembled monolayers (gold electrode with the chemically bound enzyme). Both, standard UV–Vis spectrophotometry and amperometry in a batch configuration are presented as complementary methods to study the tyrosinase enzyme kinetics, whose catecholase activity results in electroactive products (ortho-quinones). Due to higher sensitivity of amperometric detection, evident advantage in the enzyme consumption was obtained. Prepared amperometric tyrosinase biosensors were characterized using cyclic voltammetry and atomic force microscopy. The Michaelis constant values of immobilized and unbound tyrosinase (free enzyme solution) towards dopamine and catechol were compared. The apparent Michaelis constant values for immobilized tyrosinase are significantly lower than the declared value of 0.840 mmol L−1 dopamine for the unbound enzyme. The enzyme tyrosinase arranged in self-assembled monolayer serves as an efficient sensor due to low apparent Michaelis constant of 0.061 mmol L−1 dopamine and high maximum reaction velocity of 0.458 μA s−1. This fact reflects the ideal arrangement of enzyme molecules causing high availability of the binding site. Tris-glycine sodium dodecyl sulphate polyacrylamide gel electrophoresis and atomic force microscopy clarified that the protein of molecular weight 25 kDa is bound preferably on chemically modified gold electrode. A sensor prepared by the immobilization of tyrosinase on gold electrode results in higher catecholase activity towards dopamine than in case of CPE and GC electrodes, where enzyme is immobilized physically.
中文翻译:

各种固定化蘑菇酪氨酸酶的催化特性:仿生电流生物传感器未来发展的动力学研究
摘要 蘑菇酪氨酸酶通过直接嵌入电极材料(改性碳糊电极)、将交联酶聚集体掺入聚合物膜(由 Nafion® 薄层覆盖的玻璃碳电极)和使用自组装单层共价连接来固定。 (带有化学结合酶的金电极)。批量配置中的标准 UV-Vis 分光光度法和电流分析法都作为研究酪氨酸酶动力学的补充方法,其儿茶酚酶活性导致电活性产物(邻醌)。由于电流检测的灵敏度更高,在酶消耗方面获得了明显的优势。制备的电流型酪氨酸酶生物传感器使用循环伏安法和原子力显微镜进行表征。比较了固定的和未结合的酪氨酸酶(游离酶溶液)对多巴胺和儿茶酚的 Michaelis 常数值。固定化酪氨酸酶的表观 Michaelis 常数值显着低于未结合酶的 0.840 mmol L-1 多巴胺的声明值。由于 0.061 mmol L-1 多巴胺的低表观米氏常数和 0.458 μA s-1 的高最大反应速度,排列在自组装单层中的酶酪氨酸酶可用作有效的传感器。这一事实反映了酶分子的理想排列,导致结合位点的高可用性。Tris-甘氨酸十二烷基硫酸钠聚丙烯酰胺凝胶电泳和原子力显微镜表明,分子量为25 kDa的蛋白质优选结合在化学修饰的金电极上。
更新日期:2020-05-01
中文翻译:

各种固定化蘑菇酪氨酸酶的催化特性:仿生电流生物传感器未来发展的动力学研究
摘要 蘑菇酪氨酸酶通过直接嵌入电极材料(改性碳糊电极)、将交联酶聚集体掺入聚合物膜(由 Nafion® 薄层覆盖的玻璃碳电极)和使用自组装单层共价连接来固定。 (带有化学结合酶的金电极)。批量配置中的标准 UV-Vis 分光光度法和电流分析法都作为研究酪氨酸酶动力学的补充方法,其儿茶酚酶活性导致电活性产物(邻醌)。由于电流检测的灵敏度更高,在酶消耗方面获得了明显的优势。制备的电流型酪氨酸酶生物传感器使用循环伏安法和原子力显微镜进行表征。比较了固定的和未结合的酪氨酸酶(游离酶溶液)对多巴胺和儿茶酚的 Michaelis 常数值。固定化酪氨酸酶的表观 Michaelis 常数值显着低于未结合酶的 0.840 mmol L-1 多巴胺的声明值。由于 0.061 mmol L-1 多巴胺的低表观米氏常数和 0.458 μA s-1 的高最大反应速度,排列在自组装单层中的酶酪氨酸酶可用作有效的传感器。这一事实反映了酶分子的理想排列,导致结合位点的高可用性。Tris-甘氨酸十二烷基硫酸钠聚丙烯酰胺凝胶电泳和原子力显微镜表明,分子量为25 kDa的蛋白质优选结合在化学修饰的金电极上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号