当前位置:
X-MOL 学术
›
J. Inorg. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alternative modes of O2 activation in P450 and NOS enzymes are clarified by DFT modeling and resonance Raman spectroscopy.
Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2020-03-13 , DOI: 10.1016/j.jinorgbio.2020.111054 Alexandra V Soldatova 1 , Thomas G Spiro 1
Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2020-03-13 , DOI: 10.1016/j.jinorgbio.2020.111054 Alexandra V Soldatova 1 , Thomas G Spiro 1
Affiliation
|
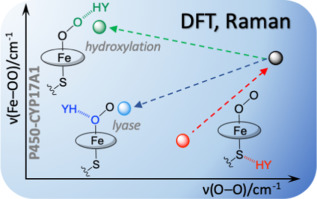
The functions of heme proteins are modulated by hydrogen bonds (H-bonds) directed at the heme-bound ligands by protein residues. When the gaseous ligands CO, NO, or O2 are bound, their activity is strongly influenced by H-bonds to their atoms. These H-bonds produce characteristic changes in the vibrational frequencies of the heme adduct, which can be monitored by resonance Raman spectroscopy and interpreted with density functional theory (DFT) computations. When the protein employs a cysteinate proximal ligand, bound O2 becomes particularly reactive, the course of the reaction being controlled by H-bonding and proton delivery. In this work, DFT modeling is used to examine the effects of H-bonding to either the terminal (Ot) or proximate (Op) atom of methylthiolate-Fe(II)porphine-O2, as well as to the thiolate S atom. H-bonds to Op produce a positive linear correlation between ν(Fe - O) and ν(O - O), because they increase the sp2 character of Op, weakening both the Fe - O and O - O bonds. H-bonds to Ot produce a negative correlation, because they increase Fe backbonding, strengthening the Fe - O but weakening the O - O bond. Available experimental data accommodate well to the computed pattern. In particular, this correspondence supports the interpretation of cytochrome P450 data by Kincaid and Sligar [M. Gregory, P.J. Mak, S.G. Sligar, J.R. Kincaid, Angew. Chem. Int. Ed. 125 (2013) 5450-5453], involving steering between hydroxylation and lyase reaction channels by differential H-bonds. Similar channeling between the first and second steps of the nitric oxide synthase reaction is likely.
中文翻译:

通过 DFT 建模和共振拉曼光谱阐明了 P450 和 NOS 酶中 O2 激活的替代模式。
血红素蛋白的功能通过蛋白质残基针对血红素结合配体的氢键(H-键)进行调节。当气态配体 CO、NO 或 O2 结合时,它们的活性受到与其原子的氢键的强烈影响。这些氢键会产生血红素加合物振动频率的特征变化,可以通过共振拉曼光谱进行监测,并通过密度泛函理论 (DFT) 计算进行解释。当蛋白质使用半胱氨酸近端配体时,结合的 O2 变得特别活跃,反应过程由氢键和质子传递控制。在这项工作中,DFT 建模用于检查氢键对硫醇盐-Fe(II)卟啉-O2 末端 (Ot) 或邻近 (Op) 原子以及硫醇盐 S 原子的影响。 Op 的氢键在 ν(Fe - O) 和 ν(O - O) 之间产生正线性相关,因为它们增加了 Op 的 sp2 特性,削弱了 Fe - O 和 O - O 键。氢键与 Ot 产生负相关,因为它们增加了 Fe 反向键合,增强了 Fe-O 键,但削弱了 O-O 键。可用的实验数据很好地适应了计算的模式。特别是,这种对应关系支持 Kincaid 和 Sligar [M. Gregory、PJ Mak、SG Sligar、JR Kincaid、Angew。化学。国际。埃德。 125 (2013) 5450-5453],涉及通过差异氢键在羟基化和裂合酶反应通道之间进行控制。一氧化氮合酶反应的第一步和第二步之间可能存在类似的通道。
更新日期:2020-03-16
中文翻译:

通过 DFT 建模和共振拉曼光谱阐明了 P450 和 NOS 酶中 O2 激活的替代模式。
血红素蛋白的功能通过蛋白质残基针对血红素结合配体的氢键(H-键)进行调节。当气态配体 CO、NO 或 O2 结合时,它们的活性受到与其原子的氢键的强烈影响。这些氢键会产生血红素加合物振动频率的特征变化,可以通过共振拉曼光谱进行监测,并通过密度泛函理论 (DFT) 计算进行解释。当蛋白质使用半胱氨酸近端配体时,结合的 O2 变得特别活跃,反应过程由氢键和质子传递控制。在这项工作中,DFT 建模用于检查氢键对硫醇盐-Fe(II)卟啉-O2 末端 (Ot) 或邻近 (Op) 原子以及硫醇盐 S 原子的影响。 Op 的氢键在 ν(Fe - O) 和 ν(O - O) 之间产生正线性相关,因为它们增加了 Op 的 sp2 特性,削弱了 Fe - O 和 O - O 键。氢键与 Ot 产生负相关,因为它们增加了 Fe 反向键合,增强了 Fe-O 键,但削弱了 O-O 键。可用的实验数据很好地适应了计算的模式。特别是,这种对应关系支持 Kincaid 和 Sligar [M. Gregory、PJ Mak、SG Sligar、JR Kincaid、Angew。化学。国际。埃德。 125 (2013) 5450-5453],涉及通过差异氢键在羟基化和裂合酶反应通道之间进行控制。一氧化氮合酶反应的第一步和第二步之间可能存在类似的通道。










































 京公网安备 11010802027423号
京公网安备 11010802027423号