当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and evaluation of targeted hypoxia-activated prodrugs applied to chondrosarcoma chemotherapy.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-03-13 , DOI: 10.1016/j.bioorg.2020.103747 Yvain Gerard 1 , Aurélien Voissière 1 , Caroline Peyrode 1 , Marie-Josephe Galmier 1 , Elise Maubert 1 , Donia Ghedira 1 , Sebastien Tarrit 1 , Vincent Gaumet 1 , Damien Canitrot 1 , Elisabeth Miot-Noirault 1 , Jean-Michel Chezal 1 , Valérie Weber 1
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-03-13 , DOI: 10.1016/j.bioorg.2020.103747 Yvain Gerard 1 , Aurélien Voissière 1 , Caroline Peyrode 1 , Marie-Josephe Galmier 1 , Elise Maubert 1 , Donia Ghedira 1 , Sebastien Tarrit 1 , Vincent Gaumet 1 , Damien Canitrot 1 , Elisabeth Miot-Noirault 1 , Jean-Michel Chezal 1 , Valérie Weber 1
Affiliation
|
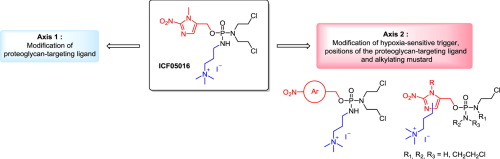
The tumor microenvironment in chondrosarcoma (CHS), a chemo- and radio-resistant cancer provides unique hallmarks for developing a chondrosarcoma targeted drug-delivery system. Tumor targeting could be achieved using a quaternary ammonium function (QA) as a ligand for aggrecan, the main high negative charged proteoglycan of the extracellular matrix of CHS, and a 2-nitroimidazole as trigger that enables hypoxia-responsive drug release. In a previous work, ICF05016 was identified as efficient proteoglycan-targeting hypoxia-activated prodrug in a human extraskeletal myxoid chondrosarcoma model in mice and a first study of the structure-activity relationship of the QA function and the alkyl linker length was conducted. Here, we report the second part of the study, namely the modification of the nitro-aromatic trigger and the position of the proteoglycan-targeting ligand at the aromatic ring as well as the nature of the alkylating mustard. Synthetic approaches have been established to functionalize the 2-nitroimidazole ring at the N-1 and C-4 positions with a terminal tertiary alkyl amine, and to perform the phosphorylation step namely through the use of an amine borane complex, leading to phosphoramide and isophosphoramide mustards and also to a phosphoramide mustard bearing four 2-chloroethyl chains. In a preliminary study using a reductive chemical activation, QA-conjugates, except the 4-nitrobenzyl one, were showed to undergo efficient cleavage with release of the corresponding mustard. However N,N,N-trimethylpropylaminium tethered to the N-1 or C-4 positions of the imidazole seemed to hamper the enzymatic reduction of the prodrugs and all tested compounds featured moderate selectivity toward hypoxic cells, likely not sufficient for application as hypoxia-activated prodrugs.
中文翻译:

设计,合成和评估用于软骨肉瘤化疗的靶向低氧激活前药。
软骨肉瘤(CHS)中的肿瘤微环境是一种化学耐药和放射耐药性癌症,为开发软骨肉瘤靶向药物递送系统提供了独特的标志。使用季铵盐功能(QA)作为聚集蛋白聚糖的配体,CHS细胞外基质的主要高负电荷蛋白聚糖和2-硝基咪唑作为触发剂,可实现低氧反应性药物释放,从而实现肿瘤靶向。在以前的工作中,ICF05016被鉴定为小鼠骨骼肌黏液样软骨肉瘤模型中靶向蛋白聚糖的低氧激活前药,并且对QA功能与烷基接头长度的结构-活性关系进行了首次研究。在这里,我们报告研究的第二部分,即硝基芳香族触发物的修饰和蛋白聚糖靶向配体在芳环上的位置以及烷基化芥菜的性质。已经建立了合成方法来用末端叔烷基胺官能化N-1和C-4位的2-硝基咪唑环,并进行磷酸化步骤,即通过使用胺硼烷络合物,从而生成磷酰胺和异磷酰胺芥末以及带有四个2-氯乙基链的磷酰胺芥末。在使用还原性化学活化的初步研究中,除4-硝基苄基外,QA共轭物被证明可有效裂解并释放相应的芥末。但是N,N
更新日期:2020-03-16
中文翻译:

设计,合成和评估用于软骨肉瘤化疗的靶向低氧激活前药。
软骨肉瘤(CHS)中的肿瘤微环境是一种化学耐药和放射耐药性癌症,为开发软骨肉瘤靶向药物递送系统提供了独特的标志。使用季铵盐功能(QA)作为聚集蛋白聚糖的配体,CHS细胞外基质的主要高负电荷蛋白聚糖和2-硝基咪唑作为触发剂,可实现低氧反应性药物释放,从而实现肿瘤靶向。在以前的工作中,ICF05016被鉴定为小鼠骨骼肌黏液样软骨肉瘤模型中靶向蛋白聚糖的低氧激活前药,并且对QA功能与烷基接头长度的结构-活性关系进行了首次研究。在这里,我们报告研究的第二部分,即硝基芳香族触发物的修饰和蛋白聚糖靶向配体在芳环上的位置以及烷基化芥菜的性质。已经建立了合成方法来用末端叔烷基胺官能化N-1和C-4位的2-硝基咪唑环,并进行磷酸化步骤,即通过使用胺硼烷络合物,从而生成磷酰胺和异磷酰胺芥末以及带有四个2-氯乙基链的磷酰胺芥末。在使用还原性化学活化的初步研究中,除4-硝基苄基外,QA共轭物被证明可有效裂解并释放相应的芥末。但是N,N











































 京公网安备 11010802027423号
京公网安备 11010802027423号