当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Potent colchicine-site ligands with improved intrinsic solubility by replacement of the 3,4,5-trimethoxyphenyl ring with a 2-methylsulfanyl-6-methoxypyridine ring.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-03-13 , DOI: 10.1016/j.bioorg.2020.103755 Raquel Álvarez 1 , Laura Aramburu 1 , Consuelo Gajate 2 , Alba Vicente-Blázquez 3 , Faustino Mollinedo 2 , Manuel Medarde 1 , Rafael Peláez 1
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-03-13 , DOI: 10.1016/j.bioorg.2020.103755 Raquel Álvarez 1 , Laura Aramburu 1 , Consuelo Gajate 2 , Alba Vicente-Blázquez 3 , Faustino Mollinedo 2 , Manuel Medarde 1 , Rafael Peláez 1
Affiliation
|
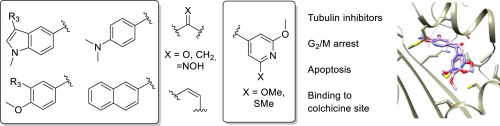
Colchicine site antimitotic agents typically suffer from low aqueous solubilities and are formulated as phosphate prodrugs of phenolic groups. These hydroxyl groups are the aim of metabolic transformations leading to resistance. There is an urgent need for more intrinsically soluble analogues lacking these hydroxyl groups. The 3,4,5-trimethoxyphenyl ring of combretastatin A-4 is a liability in terms of solubility but it is considered essential for high cytotoxic and tubulin polymerization inhibitory (TPI) activity. We have synthesized 36 new analogues of combretastatin A-4 replacing the trimethoxyphenyl moiety with more polar pyridine based moieties, measured their aqueous solubility, and studied their anti-proliferative effects against 3 human cancer cell lines. We show here that pyridine rings can be successful replacements for the trimethoxyphenyl ring, resulting in potent and more soluble analogues. The more straightforward replacement, a 2,6-dimethoxypyridine ring led to inactive analogues, but a 2-methoxy-6-methylsulfanylpyridine moiety led to active analogues when combined with different B rings. This replacement led to potent cytotoxic activity against sensitive human cancer cell lines due to tubulin inhibition, as shown by cell cycle analysis, confocal microscopy, and tubulin polymerization inhibitory activity studies. Cell cycle analysis also showed apoptotic responses following treatment. Docking studies suggested binding at the colchicine site of tubulin and provided a good agreement with the observed SAR. A 2-methoxy-6-methylsulfanylpyridine moiety is a good trimethoxyphenyl ring replacement for the development of new colchicine site ligands.
中文翻译:

通过用2-甲基硫烷基-6-甲氧基吡啶环取代3,4,5-三甲氧基苯基环,具有改善的固有溶解度的强秋水仙碱位配体。
秋水仙碱位点抗有丝分裂剂通常具有低的水溶性,并被配制成酚基的磷酸盐前药。这些羟基是导致抗性的代谢转化的目的。迫切需要缺少这些羟基的更易溶于水的类似物。康布雷他汀A-4的3,4,5-三甲氧基苯基环在溶解度方面是一个责任,但它被认为对高细胞毒性和微管蛋白聚合抑制(TPI)活性至关重要。我们已经合成了康布雷他汀A-4的36个新类似物,用更多的极性吡啶基部分取代了三甲氧基苯基部分,测量了它们的水溶性,并研究了其对3种人类癌细胞系的抗增殖作用。我们在这里显示吡啶环可以成功取代三甲氧基苯基环,从而产生有效且更易溶的类似物。更直接的替换是,当与不同的B环结合时,2,6-二甲氧基吡啶环导致失活的类似物,但是2-甲氧基-6-甲基磺酰氨吡啶部分导致活性的类似物。如细胞周期分析,共聚焦显微镜和微管蛋白聚合抑制活性研究所示,由于微管蛋白抑制作用,这种替代导致了对敏感的人类癌细胞系的有效细胞毒活性。细胞周期分析还显示了治疗后的凋亡反应。对接研究表明在微管蛋白的秋水仙碱位点具有结合力,并与观察到的SAR有很好的一致性。
更新日期:2020-03-16
中文翻译:

通过用2-甲基硫烷基-6-甲氧基吡啶环取代3,4,5-三甲氧基苯基环,具有改善的固有溶解度的强秋水仙碱位配体。
秋水仙碱位点抗有丝分裂剂通常具有低的水溶性,并被配制成酚基的磷酸盐前药。这些羟基是导致抗性的代谢转化的目的。迫切需要缺少这些羟基的更易溶于水的类似物。康布雷他汀A-4的3,4,5-三甲氧基苯基环在溶解度方面是一个责任,但它被认为对高细胞毒性和微管蛋白聚合抑制(TPI)活性至关重要。我们已经合成了康布雷他汀A-4的36个新类似物,用更多的极性吡啶基部分取代了三甲氧基苯基部分,测量了它们的水溶性,并研究了其对3种人类癌细胞系的抗增殖作用。我们在这里显示吡啶环可以成功取代三甲氧基苯基环,从而产生有效且更易溶的类似物。更直接的替换是,当与不同的B环结合时,2,6-二甲氧基吡啶环导致失活的类似物,但是2-甲氧基-6-甲基磺酰氨吡啶部分导致活性的类似物。如细胞周期分析,共聚焦显微镜和微管蛋白聚合抑制活性研究所示,由于微管蛋白抑制作用,这种替代导致了对敏感的人类癌细胞系的有效细胞毒活性。细胞周期分析还显示了治疗后的凋亡反应。对接研究表明在微管蛋白的秋水仙碱位点具有结合力,并与观察到的SAR有很好的一致性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号