Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-03-12 , DOI: 10.1016/j.jcat.2020.02.011 Binbin Nian , Guangfu Liao , Ying Song , YingZhu Su , Chen Cao , Yuanfa Liu
|
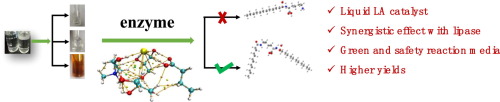
To improve their catalytic activity, herein, three types of metal chloride hydrate were introduced into pure choline chloride-glycerol (CGly) for fabricating new three constituents-natural deep eutectic solvents (3c-NADESs) with enhanced Lewis acidity. Attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) confirmed that the Lewis acidity of CGly was significantly improved due to the addition of metal chloride hydrates. The catalytic performance of these new-designed 3c-NADESs was characterized via the amidation reaction of lauric acid and lysine. Compared with pure CGly, a significant enhancement of the yield of Gemini lauroyl lysine (GLL) was obtained. Interestingly, the control experiments suggested that there was an apparent synergistic effect between Lipase immobilized on acrylic resin from Candida antarctica (CALB) and NADESs. Quantum calculation studies suggested fatty acid and amino acid were activated by chloride ions via multiple hydrogen-bonding interactions, resulting in the enhancement of electron-attracting capacity of fatty acid and electron-loss ability of amino acid. The frontier molecular orbits analysis provided us with a cogent evidence that chloride ions activated both lysine and lauric acid and made the reaction easier to be performed. The intrinsic reaction coordinates (IRC) study indicated chloride ions act as a “freighter”, which transported the hydrogen atom of amino group to the hydroxyl group. This in-depth study may shed light on developing sustainable and efficient strategies of amidation and other nucleophilic or electrophilic reactions.
中文翻译:

离子氢键相互作用控制亲电性和亲核性:酰胺酶和天然深共熔溶剂在酰胺化反应中协同催化作用的机理研究
为了提高它们的催化活性,在本文中,将三种类型的金属氯化物水合物引入纯的氯化胆碱-甘油(CGly)中,以制备新的三种成分-具有增强的路易斯酸性的天然深共晶溶剂(3c-NADES)。衰减全反射傅立叶变换红外光谱(ATR-FTIR)证实,由于添加了金属氯化物水合物,CGly的路易斯酸度得到了显着改善。这些新设计的3c-NADES的催化性能通过月桂酸和赖氨酸的酰胺化反应来表征。与纯CGly相比,双子叶月桂酰赖氨酸(GLL)的产量显着提高。有趣的是,对照实验表明,固定在脂肪酶上的脂酶与丙烯酸树脂之间存在明显的协同作用。南极假丝酵母(CALB)和NADES。量子计算研究表明,脂肪酸和氨基酸通过多种氢键相互作用被氯离子活化,从而增强了脂肪酸的电子吸引能力和氨基酸的电子损失能力。前沿的分子轨道分析为我们提供了有力的证据,表明氯离子激活了赖氨酸和月桂酸,并使反应更容易进行。内在反应坐标(IRC)研究表明,氯离子充当“增重剂”,将氨基的氢原子转运至羟基。这项深入的研究可能会为制定可持续和有效的酰胺化以及其他亲核或亲电子反应策略提供启示。











































 京公网安备 11010802027423号
京公网安备 11010802027423号