Chemical Physics ( IF 2.3 ) Pub Date : 2020-03-14 , DOI: 10.1016/j.chemphys.2020.110754 Renáta Homlok , Viktória Mile , Erzsébet Takács , Gábor Járvás , Szabolcs Góger , László Wojnárovits
|
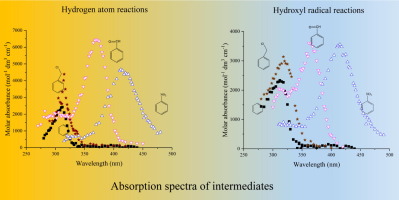
The reactions of hydrogen atoms and hydroxyl radicals with benzene and 10 monosubstituted benzenes were investigated by measuring transient absorption spectra, calculating Gibbs free energies, absorption spectra of intermediates formed and determining the rate constants of H• reactions. The basic reaction is addition to the aromatic ring forming cyclohexadienyl type radicals. Most radical exhibit absorption spectra with maxima in the 300-340 nm range, these maxima for molecules with strong electron withdrawing groups are shifted to 340-420 nm. The spectra of H• and •OH adducts are similar suggesting similar intermediates. In substituted benzenes the absorption band is due to several isomers. Ortho and para additions are important in reaction with all molecules studied. Both, H• and •OH can react with the –Cl, –Br and –NO2 substituents in exchange reaction without cyclohexadienyl radical. •OH reacts with the aromatic ring of benzyl chloride in radical addition, while H• eliminates Cl from the substituent.
中文翻译:

水溶液中简单芳香族分子的氢原子和羟基自由基反应的比较
通过测量瞬态吸收光谱,计算吉布斯自由能,形成的中间体的吸收光谱并确定H •反应的速率常数,研究了氢原子和羟基与苯和10个单取代苯的反应。碱性反应是加成芳环形成环己二烯基型自由基。大多数自由基显示出最大300-340 nm范围内的吸收光谱,具有强吸电子基团的分子的最大吸收谱移至340-420 nm。H •和• OH加合物的光谱相似,表明中间体相似。在取代的苯中,吸收带归因于几种异构体。邻位和对位添加物对于与所有研究分子的反应都很重要。H •和• OH均可与–Cl,–Br和–NO 2取代基发生交换反应而没有环己二烯基。• OH在自由基加成中与苄基氯的芳环反应,而• H •从取代基中消除Cl。



























 京公网安备 11010802027423号
京公网安备 11010802027423号