当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ultrasound-responsive polymersomes capable of endosomal escape for efficient cancer therapy.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-13 , DOI: 10.1016/j.jconrel.2020.03.013 Ping Wei 1 , Min Sun 2 , Bo Yang 2 , Jiangang Xiao 2 , Jianzhong Du 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-13 , DOI: 10.1016/j.jconrel.2020.03.013 Ping Wei 1 , Min Sun 2 , Bo Yang 2 , Jiangang Xiao 2 , Jianzhong Du 1
Affiliation
|
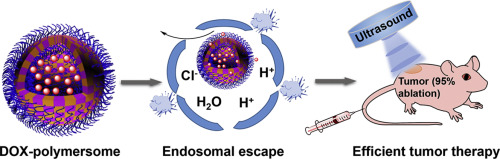
Stimuli-responsive anticancer drug delivery vehicles have attracted increasing attention in nanomedicine. However, controlled drug release in vivo is still an important challenge, as traditional stimuli lack maneuverability. To solve this problem, we designed an ultrasound and pH-responsive polymersome by self-assembly of poly(ethylene oxide)-block-poly(2-(diethylamino)ethyl methacrylate)-stat-poly(methoxyethyl methacrylate) [PEO-b-P(DEA-stat-MEMA)], where PEO acts as the corona-forming block, DEA acts as the endosomal escape segment, and MEMA acts as the ultrasound-responsive segment. This strategy combines the advantages of noninvasive ultrasonic stimulus which can be applied from outside to any organ regardless of depth, and the weakly acidic microenvironment of tumor tissue. In vitro experiments confirmed excellent endosomal escape ability, on-demand drug release behavior, low cytotoxicity, and high intracellular delivery efficiency of polymersomes. In vivo antitumor tests revealed that in the presence of sonication, the anticancer drug was released at an accelerated rate from these ultrasound-responsive polymersomes, and the DOX-loaded polymersomes + sonication group significantly inhibited tumor growth (95% reduction in tumor mass) without any side effects. Overall, this ultrasound-responsive polymersome provides us with a fresh insight into designing next-generation stimuli-responsive drug carriers with better maneuverability and higher chemotherapeutic efficiency.
中文翻译:

超声响应聚合物囊泡能够逃逸内体,实现有效的癌症治疗。
刺激响应性抗癌药物输送载体在纳米医学领域引起了越来越多的关注。然而,由于传统刺激缺乏可操作性,体内受控药物释放仍然是一个重要的挑战。为了解决这个问题,我们通过聚(环氧乙烷)-嵌段-聚(2-(二乙氨基)甲基丙烯酸乙酯)-stat-聚(甲基丙烯酸甲氧基乙酯)[PEO-bP( DEA-stat-MEMA)],其中 PEO 充当冠形成块,DEA 充当内体逃逸段,MEMA 充当超声响应段。该策略结合了无创超声刺激的优点,可以从外部施加到任何器官,无论深度如何,以及肿瘤组织的弱酸性微环境。体外实验证实了聚合物囊泡具有优异的内体逃逸能力、按需药物释放行为、低细胞毒性和高细胞内递送效率。体内抗肿瘤测试表明,在超声处理的情况下,抗癌药物从这些超声响应聚合物囊泡中加速释放,并且负载 DOX 的聚合物囊泡 + 超声处理组显着抑制肿瘤生长(肿瘤质量减少 95%),且不产生任何副作用。任何副作用。总体而言,这种超声响应聚合物囊泡为我们设计具有更好可操作性和更高化疗效率的下一代刺激响应药物载体提供了新的见解。
更新日期:2020-03-16
中文翻译:

超声响应聚合物囊泡能够逃逸内体,实现有效的癌症治疗。
刺激响应性抗癌药物输送载体在纳米医学领域引起了越来越多的关注。然而,由于传统刺激缺乏可操作性,体内受控药物释放仍然是一个重要的挑战。为了解决这个问题,我们通过聚(环氧乙烷)-嵌段-聚(2-(二乙氨基)甲基丙烯酸乙酯)-stat-聚(甲基丙烯酸甲氧基乙酯)[PEO-bP( DEA-stat-MEMA)],其中 PEO 充当冠形成块,DEA 充当内体逃逸段,MEMA 充当超声响应段。该策略结合了无创超声刺激的优点,可以从外部施加到任何器官,无论深度如何,以及肿瘤组织的弱酸性微环境。体外实验证实了聚合物囊泡具有优异的内体逃逸能力、按需药物释放行为、低细胞毒性和高细胞内递送效率。体内抗肿瘤测试表明,在超声处理的情况下,抗癌药物从这些超声响应聚合物囊泡中加速释放,并且负载 DOX 的聚合物囊泡 + 超声处理组显着抑制肿瘤生长(肿瘤质量减少 95%),且不产生任何副作用。任何副作用。总体而言,这种超声响应聚合物囊泡为我们设计具有更好可操作性和更高化疗效率的下一代刺激响应药物载体提供了新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号