当前位置:
X-MOL 学术
›
Anal. Chim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction between Antifreeze Protein and Ice Crystal Facet Evaluated by Ice-Channel Electrophoretic Measurements of Threshold Electric Field Strength
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.aca.2020.03.025 Arinori Inagawa , Nobuo Uehara , Tetsuo Okada
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.aca.2020.03.025 Arinori Inagawa , Nobuo Uehara , Tetsuo Okada
|
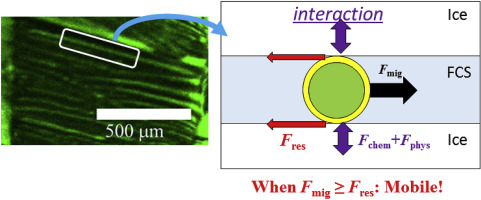
The chemical interaction between antifreeze proteins (AFPs) and ice crystals is evaluated via electrophoresis of AFP-anchored microparticles in fluidic channels formed in frozen aqueous sucrose. Straight fluidic channels are created in a flat glass chamber connecting two Ag/AgCl electrodes. This configuration allows us to estimate an electric field strength exerted on probe particles migrating along the channel. When the channel width is comparable to the particle size, the particle is immobile because of the resistance force induced by the interaction with the ice wall. However, when the overall electrophoretic force surpasses the resistance force, the microsphere starts to migrate. From the threshold electric field strengths determined for unmodified and AFP-modified particles, the resistance forces for the chemical interaction between AFPs and ice wall are estimated.
中文翻译:

通过阈值电场强度的冰通道电泳测量评估防冻蛋白和冰晶面之间的相互作用
抗冻蛋白 (AFP) 和冰晶之间的化学相互作用通过 AFP 锚定微粒在冷冻蔗糖水溶液中形成的流体通道中的电泳进行评估。在连接两个 Ag/AgCl 电极的平板玻璃室中创建直流通道。这种配置使我们能够估计施加在沿通道迁移的探针粒子上的电场强度。当通道宽度与粒径相当时,由于与冰壁相互作用引起的阻力,颗粒是不动的。然而,当整体电泳力超过阻力时,微球开始迁移。根据为未改性和 AFP 改性的颗粒确定的阈值电场强度,
更新日期:2020-05-01
中文翻译:

通过阈值电场强度的冰通道电泳测量评估防冻蛋白和冰晶面之间的相互作用
抗冻蛋白 (AFP) 和冰晶之间的化学相互作用通过 AFP 锚定微粒在冷冻蔗糖水溶液中形成的流体通道中的电泳进行评估。在连接两个 Ag/AgCl 电极的平板玻璃室中创建直流通道。这种配置使我们能够估计施加在沿通道迁移的探针粒子上的电场强度。当通道宽度与粒径相当时,由于与冰壁相互作用引起的阻力,颗粒是不动的。然而,当整体电泳力超过阻力时,微球开始迁移。根据为未改性和 AFP 改性的颗粒确定的阈值电场强度,











































 京公网安备 11010802027423号
京公网安备 11010802027423号