Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2020-03-13 , DOI: 10.1016/j.jechem.2020.03.013 Luis Sandoval-Diaz , Milivoj Plodinec , Danail Ivanov , Stéphane Poitel , Adnan Hammud , Hannah C. Nerl , Robert Schlögl , Thomas Lunkenbein
|
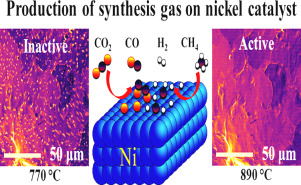
Synthesis gas, composed of H2 and CO, is an important fuel which serves as feedstock for industrially relevant processes, such as methanol or ammonia synthesis. The efficiency of these reactions depends on the H2: CO ratio, which can be controlled by a careful choice of reactants and catalyst surface chemistry. Here, using a combination of environmental scanning electron microscopy (ESEM) and online mass spectrometry, direct visualization of the surface chemistry of a Ni catalyst during the production of synthesis gas was achieved for the first time. The insertion of a homebuilt quartz tube reactor in the modified ESEM chamber was key to success of the setup. The nature of chemical dynamics was revealed in the form of reversible oxide-metal phase transitions and surface transformations which occurred on the performing catalyst. The oxide-metal phase transitions were found to control the production of synthesis gas in the temperature regime between 700 and 900 °C in an atmosphere relevant for dry reforming of methane (DRM, CO2: CH4 =0.75). This was confirmed using high resolution transmission electron microscopy imaging, electron energy loss spectroscopy, thermal analysis, and C18O2 labelled experiments. Our dedicated operando approach of simultaneously studying the surface processes of a catalyst and its activity allowed to uncover how phase transitions can steer catalytic reactions.
中文翻译:

可视化在Ni催化剂上合成气生产中氧化物-金属相变的重要性
由H 2和CO组成的合成气是一种重要的燃料,可用作工业相关过程(例如甲醇或氨合成)的原料。这些反应的效率取决于H 2:CO比,可以通过仔细选择反应物和催化剂表面化学来控制。在此,结合使用环境扫描电子显微镜(ESEM)和在线质谱分析技术,首次实现了在合成气生产过程中直接观察镍催化剂的表面化学性质。在改良的ESEM室中插入一个自制的石英管反应器是成功安装的关键。化学动力学的性质以在可运行的催化剂上发生的可逆的氧化物-金属相变和表面转变的形式揭示。发现氧化物-金属相变可在与甲烷干重整相关的气氛(DRM,CO 2:CH 4= 0.75)。使用高分辨率透射电子显微镜成像,电子能量损失谱,热分析和C 18 O 2标记的实验证实了这一点。我们专用的操作方法同时研究催化剂的表面过程及其活性,从而揭示了相变如何引导催化反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号