Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
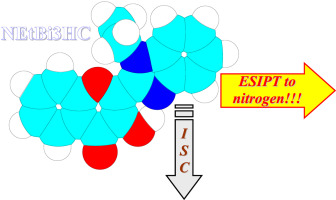
N-ethyl substituted 2-benzimidazolyl-3-hydroxychromone: Atypical to highly fluorescent dyes of flavonol series excited state intramolecular proton transfer to nitrogen
Journal of Luminescence ( IF 3.3 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.jlumin.2020.117206 Andrii Yu Chumak , Yelizaveta O. Denysieva , Oleksii O. Kolomoitsev , Volodymyr M. Kotlyar , Elena H. Shvets , Andrey O. Doroshenko
Journal of Luminescence ( IF 3.3 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.jlumin.2020.117206 Andrii Yu Chumak , Yelizaveta O. Denysieva , Oleksii O. Kolomoitsev , Volodymyr M. Kotlyar , Elena H. Shvets , Andrey O. Doroshenko
|
Abstract New derivative of 3-hydroxychromone possessing N-ethyl substituted benzimidazole moiety was specially synthesized with the aim to switch the excited state intramolecular proton transfer (ESIPT) to heterocyclic nitrogen atom, this ESIPT direction is generally less typical to fluorescent compounds of flavonol series. Bulky ethyl group makes conformation with intramolecular hydrogen bond to carbonyl group oxygen atom unfavorable in the ground state. We expected the same situation remaining in the excited state. Photophysics of the title compound was studied both experimentally and theoretically. Unlike weakly fluorescent 3-hydroxychromones with 2′-pyridyl or 2′-quinolyl heterocycles in position 2, the title compound demonstrates unexpectedly high fluorescence quantum yields in solvents of low-to-intermediate polarity. We consider the higher rate of intramolecular proton transfer reaction, in comparison with concurring radiationless intersystem crossing (ISC) process with participation of singlet and triplet nπ* levels of carbonyl group, as the main reason of bright fluorescence of title compound.
中文翻译:

N-乙基取代的 2-苯并咪唑基-3-羟基色酮:黄酮醇系列激发态分子内质子转移至氮的非典型至高荧光染料
摘要 为了将激发态分子内质子转移(ESIPT)转换为杂环氮原子,专门合成了具有N-乙基取代的苯并咪唑部分的3-羟基色酮的新衍生物,该ESIPT方向对于黄酮醇系列的荧光化合物通常不太典型。庞大的乙基使分子内氢键与羰基氧原子的构象在基态不利。我们预计同样的情况会保持在激发态。通过实验和理论研究了标题化合物的光物理学。与在 2 位具有 2'-吡啶基或 2'-喹啉基杂环的弱荧光 3-羟基色酮不同,标题化合物在低至中等极性的溶剂中表现出出乎意料的高荧光量子产率。
更新日期:2020-07-01
中文翻译:

N-乙基取代的 2-苯并咪唑基-3-羟基色酮:黄酮醇系列激发态分子内质子转移至氮的非典型至高荧光染料
摘要 为了将激发态分子内质子转移(ESIPT)转换为杂环氮原子,专门合成了具有N-乙基取代的苯并咪唑部分的3-羟基色酮的新衍生物,该ESIPT方向对于黄酮醇系列的荧光化合物通常不太典型。庞大的乙基使分子内氢键与羰基氧原子的构象在基态不利。我们预计同样的情况会保持在激发态。通过实验和理论研究了标题化合物的光物理学。与在 2 位具有 2'-吡啶基或 2'-喹啉基杂环的弱荧光 3-羟基色酮不同,标题化合物在低至中等极性的溶剂中表现出出乎意料的高荧光量子产率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号