Electrochimica Acta ( IF 5.5 ) Pub Date : 2020-03-14 , DOI: 10.1016/j.electacta.2020.136063 Jorge Montero , Daniel Arenas-Esteban , David Ávila-Brande , Elizabeth Castillo-Martínez , Silvia Licoccia , Javier Carretero-González
|
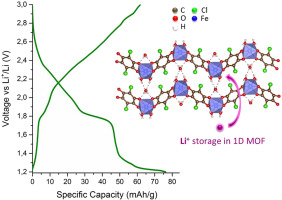
The lithium ion storage properties of a series of metal-organic frameworks (MOFs) with formula {[M(L)(H2O)2]H2O}n and [M(CA)(Pyz)]n (where L refers to the tetraoxolene ligands: CA = chloranilate and DHBQ = dihydroxybenzoquinone; Pyz = pyrazine; M = Fe and Mn) and exhibiting a 1D and 2D structure, respectively, have been studied. The 1D MOFs ({[M(L)(H2O)2]H2O}n) show higher reversible capacity values for lithium ion insertion with respect to 2D structures containing two organic ligands (M(CA)(Pyz)]n). The gravimetric capacity for the 1D Fe-CA MOF is 75 mAh/g at 2.16 mA/g (∼ 1 lithium atom per formula unit) higher than for the Mn complexes which is 65 mAh/g at 2.12 mA/g, though isostructural. Lithium ion insertion in the 1D Mn-CA chains takes place at 2.4 V vs. Li+/Li which is ∼700 mV higher than what is recorded for the Fe analogue. This result is most probably due to much more stable d5 electronic configuration of Mn2+ than d6 of Fe2+ in its isostructural Fe-based framework analogue. The 1D Fe-DHBQ capacity is higher than its manganese analogue 75 mAh/g at 2.5 mA/g (0.8 lithiums) against 40 mAh/g. In general, the high voltages of reaction in these 1D MOFs suggest that they involve the participation of the ligand on the redox processes along with the reduction of the transition metal if any. In fact, the potential of ion insertion changed depending on the metal. This fact along with the absence of evidence of conversion reaction by x-ray diffraction of cycled electrodes suggests that the charge delocalization may be all along the metal-ligand molecular framework participating as a whole hybrid unit in the lithium storage.
中文翻译:

一维和二维氧化还原活性金属-有机框架中的锂离子存储
一系列具有式{[M(L)(H 2 O)2 ] H 2 O} n和[M(CA)(Pyz)] n的金属有机骨架(MOF)的锂离子存储特性指四氧戊烯配体:CA =氯苯甲酸酯,DHBQ =二羟基苯醌; Pyz =吡嗪; M = Fe和Mn),并且分别显示了1D和2D结构。一维MOF({[M(L)(H 2 O)2 ] H 2相对于包含两个有机配体(M(CA)(Pyz)] n)的2D结构,O} n)显示出更高的锂离子可逆容量值。一维Fe-CA MOF在2.16 mA / g时的重量容量为75 mAh / g(每个配方单元约1个锂原子),比Mn络合物在2.12 mA / g时的重量容量为65 mAh / g,尽管具有同构结构。一维Mn-CA链中的锂离子插入发生在2.4 V vs. Li + / Li的情况下,这比Fe类似物的记录高出约700 mV。该结果很可能是由于Mn 2+的d 5电子构型比Fe 2+的d 6更稳定在其基于铁的同构框架类似物中。一维Fe-DHBQ容量在2.5 mA / g(0.8锂)下比其锰类似物75 mAh / g高40 mAh / g。通常,这些一维MOF中的高反应电压表明它们涉及配体参与氧化还原过程以及过渡金属(如果有)的还原。实际上,离子插入的电势根据金属而变化。这一事实以及缺乏通过循环电极的X射线衍射进行转化反应的证据表明,电荷离域可能一直沿整个锂-配体单元的整个混合单元参与。











































 京公网安备 11010802027423号
京公网安备 11010802027423号