Tetrahedron ( IF 2.1 ) Pub Date : 2020-03-13 , DOI: 10.1016/j.tet.2020.131113 Felicity Frank , Laura Manzoli Alice , Philipp Mauker , Abdulrahman A. Alsimaree , Paul Gordon Waddell , Michael Richard Probert , Thomas James Penfold , Julian Gary Knight , Michael John Hall
|
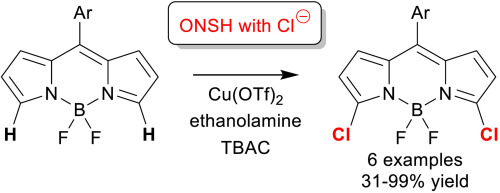
Regioselective halogenation is often a key step in the formation of substituted 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) fluorophores, through the enablement of subsequent downstream C–C or C-X bond forming steps via SNAr or metal catalyzed cross-coupling reactions. Classical SEAr halogenation of unsubstituted BODIPYs results in 2/6-substitution, precluding easy access to 3/5-halogenated BODIPYs. Herein we present our development of a 3,5-dihalogenation reaction of unsubstituted BODIPYs, via a double oxidative nucleophilic substitution of hydrogen with chloride. Reaction of a range of meso-aryl, but otherwise unsubstituted, BODIPYs with stoichiometric Cu(OTf)2 in the presence of ethanolamine and tetrabutylammonium chloride gives high isolated yields of the corresponding 3,5-dichlorinated BODIPYs, facilitating access to these valuable synthetic intermediates.
中文翻译:

3,5-二氯-4,4-二氟-4-硼-3a,4a-二氮杂的合成小号经由铜-indacenes(BODIPYs)(OTF)2介导的氧化亲核取代氢的氯化
通过进行后续的下游C–C或CX键形成步骤,区域选择性卤化通常是形成取代的4,4-二氟-4-硼3a,4a-二氮杂s-茚并四烯(BODIPY)荧光团的关键步骤通过S N Ar或金属催化的交叉偶联反应。古典小号È的2/6-取代或未取代的BODIPYs结果的Ar卤化,排除至3/5-卤代BODIPYs方便地访问。在这里,我们介绍了通过氢与氯化物的双氧化亲核取代,我们未取代的BODIPYs的3,5-二卤代反应的发展。一定范围的内消旋芳基与其他化学计量的Cu(OTf)2反应 在乙醇胺和氯化四丁基铵存在下,分离出相应的3,5-二氯代BODIPYs的产率很高,从而有助于获得这些有价值的合成中间体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号