Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-03-13 , DOI: 10.1016/j.tetlet.2020.151822 Dinesh Chandra , Saumya Verma , Chandra Bhan Pandey , Ajay K. Yadav , Puneet Kumar , Bhoopendra Tiwari , Jawahar L. Jat
|
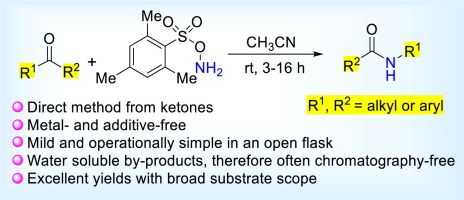
The Beckmann rearrangement is a versatile method for the preparation of secondary amides from ketones via oxime intermediates and has been widely used in the synthesis of bioactive natural products and pharmaceuticals. Herein, we have developed a highly efficient direct method for the preparation of secondary amides and lactams from ketones using O-(mesitylsulfonyl)hydroxylamine (MSH). The reactions proceed rapidly at room temperature under mild condition without requiring any additive, and tolerate multiple functional groups. A simple aqueous work-up often furnished the products in excellent yield with high purity.
中文翻译:

使用O-(异磺酰基)羟胺通过Beckmann重排从酮直接合成仲酰胺。
贝克曼重排是一种通过肟中间体由酮制备仲酰胺的通用方法,已被广泛用于合成生物活性天然产物和药物。本文中,我们已经开发出一种高效的直接方法,可使用O-(甲磺酰基磺酰基)羟胺(MSH)从酮制备仲酰胺和内酰胺。反应在室温和温和条件下快速进行,不需要任何添加剂,并且可以耐受多个官能团。简单的水后处理通常以优异的产率提供高纯度的产品。









































 京公网安备 11010802027423号
京公网安备 11010802027423号