当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Strategic Incorporation of Polarity in Heme-Displacing Inhibitors of Indoleamine-2,3-dioxygenase-1 (IDO1)
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-03-10 , DOI: 10.1021/acsmedchemlett.0c00010 Catherine White , Meredeth A. McGowan , Hua Zhou , Nunzio Sciammetta , Xavier Fradera , Jongwon Lim , Elizabeth M. Joshi , Christine Andrews , Elliott B. Nickbarg , Phillip Cowley , Sarah Trewick , Martin Augustin 1 , Konstanze von Köenig 1 , Charles A. Lesburg , Karin Otte , Ian Knemeyer , Hyun Woo , Wensheng Yu , Mangeng Cheng , Peter Spacciapoli , Prasanthi Geda , Xuelei Song , Nadya Smotrov , Patrick Curran , Mee Ra Heo , Pravien Abeywickrema , J. Richard Miller , David Jonathan Bennett , Yongxin Han
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-03-10 , DOI: 10.1021/acsmedchemlett.0c00010 Catherine White , Meredeth A. McGowan , Hua Zhou , Nunzio Sciammetta , Xavier Fradera , Jongwon Lim , Elizabeth M. Joshi , Christine Andrews , Elliott B. Nickbarg , Phillip Cowley , Sarah Trewick , Martin Augustin 1 , Konstanze von Köenig 1 , Charles A. Lesburg , Karin Otte , Ian Knemeyer , Hyun Woo , Wensheng Yu , Mangeng Cheng , Peter Spacciapoli , Prasanthi Geda , Xuelei Song , Nadya Smotrov , Patrick Curran , Mee Ra Heo , Pravien Abeywickrema , J. Richard Miller , David Jonathan Bennett , Yongxin Han
Affiliation
|
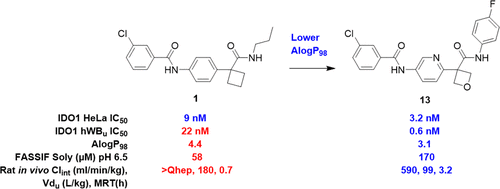
Indoleamine-2,3-dioxygenase-1 (IDO1) has emerged as a target of significant interest to the field of cancer immunotherapy, as the upregulation of IDO1 in certain cancers has been linked to host immune evasion and poor prognosis for patients. In particular, IDO1 inhibition is of interest as a combination therapy with immune checkpoint inhibition. Through an Automated Ligand Identification System (ALIS) screen, a diamide class of compounds was identified as a promising lead for the inhibition of IDO1. While hit 1 possessed attractive cell-based potency, it suffered from a significant right-shift in a whole blood assay, poor solubility, and poor pharmacokinetic properties. Through a physicochemical property-based approach, including a focus on lowering AlogP98 via the strategic introduction of polar substitution, compound 13 was identified bearing a pyridyl oxetane core. Compound 13 demonstrated improved whole blood potency and solubility, and an improved pharmacokinetic profile resulting in a low predicted human dose.
中文翻译:

在吲哚胺-2,3-双加氧酶-1(IDO1)的血红素置换抑制剂中策略性整合极性
吲哚胺-2,3-二加氧酶-1(IDO1)已成为癌症免疫治疗领域的重要目标,因为某些癌症中IDO1的上调与宿主免疫逃逸和患者预后不良有关。特别地,IDO1抑制作为与免疫检查点抑制的组合疗法受到关注。通过自动配体识别系统(ALIS)筛选,二酰胺类化合物被鉴定为抑制IDO1的有前途的先导。虽然命中1具有有吸引力的基于细胞的潜能,但在全血测定中却出现明显的右移,溶解度差和药物动力学特性差的情况。通过基于物理化学性质的方法,包括重点降低AlogP 98通过策略性引入极性取代,鉴定出带有吡啶基氧杂环丁烷核的化合物13。化合物13显示出改善的全血效力和溶解性,以及改善的药代动力学特征,导致较低的预期人剂量。
更新日期:2020-04-23
中文翻译:

在吲哚胺-2,3-双加氧酶-1(IDO1)的血红素置换抑制剂中策略性整合极性
吲哚胺-2,3-二加氧酶-1(IDO1)已成为癌症免疫治疗领域的重要目标,因为某些癌症中IDO1的上调与宿主免疫逃逸和患者预后不良有关。特别地,IDO1抑制作为与免疫检查点抑制的组合疗法受到关注。通过自动配体识别系统(ALIS)筛选,二酰胺类化合物被鉴定为抑制IDO1的有前途的先导。虽然命中1具有有吸引力的基于细胞的潜能,但在全血测定中却出现明显的右移,溶解度差和药物动力学特性差的情况。通过基于物理化学性质的方法,包括重点降低AlogP 98通过策略性引入极性取代,鉴定出带有吡啶基氧杂环丁烷核的化合物13。化合物13显示出改善的全血效力和溶解性,以及改善的药代动力学特征,导致较低的预期人剂量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号