当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pyrrolyl Pyrazoles as Non-Diketo Acid Inhibitors of the HIV-1 Ribonuclease H Function of Reverse Transcriptase.
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-03-05 , DOI: 10.1021/acsmedchemlett.9b00617 Antonella Messore 1 , Angela Corona 2 , Valentina Noemi Madia 1 , Francesco Saccoliti 1 , Valeria Tudino 1 , Alessandro De Leo 1 , Luigi Scipione 1 , Daniela De Vita 1 , Giorgio Amendola 3 , Salvatore Di Maro 3 , Ettore Novellino 4 , Sandro Cosconati 3 , Mathieu Métifiot 5 , Marie-Line Andreola 5 , Piera Valenti 6 , Francesca Esposito 2 , Nicole Grandi 2 , Enzo Tramontano 2 , Roberta Costi 1 , Roberto Di Santo 1
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-03-05 , DOI: 10.1021/acsmedchemlett.9b00617 Antonella Messore 1 , Angela Corona 2 , Valentina Noemi Madia 1 , Francesco Saccoliti 1 , Valeria Tudino 1 , Alessandro De Leo 1 , Luigi Scipione 1 , Daniela De Vita 1 , Giorgio Amendola 3 , Salvatore Di Maro 3 , Ettore Novellino 4 , Sandro Cosconati 3 , Mathieu Métifiot 5 , Marie-Line Andreola 5 , Piera Valenti 6 , Francesca Esposito 2 , Nicole Grandi 2 , Enzo Tramontano 2 , Roberta Costi 1 , Roberto Di Santo 1
Affiliation
|
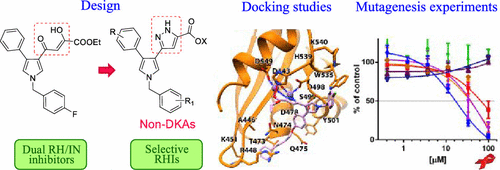
Due to the biological liability of diketo acid (DKA) chain, we transferred this element of our previously reported anti-HIV-1 pyrrolyl derivatives to a non-DKA scaffold, obtaining a series of pyrrolyl-pyrazole carboxylic acids as new RNase H inhibitors. Among the newly synthesized derivatives, oxyphenylpyrrolyl-pyrazoles demonstrated inhibitory activities within the low micromolar/submicromolar range with compound 11b being the most potent. Interestingly, all tested compounds showed up to 2 orders of magnitude of selectivity for RNase H vs integrase. Docking studies within the RNase H catalytic site, coupled with site-directed mutagenesis, showed the key structural features that could confer the ability to establish specific interactions within RNase H. Furthermore, they proved the ability of our compounds to interact with amino acids highly conserved among HIV-1 subspecies isolated among patients carrying drug-resistant variants. In the end, the newly discovered pyrazole carboxylic acid derivatives feature promising serum stability with respect to their corresponding DKAs.
中文翻译:

吡咯基吡唑类化合物是HIV-1核糖核酸酶H逆转录酶功能的非二酮酸抑制剂。
由于二酮酸(DKA)链的生物责任,我们将先前报道的抗HIV-1吡咯基衍生物的这一元素转移到了非DKA支架上,获得了一系列吡咯基-吡唑羧酸作为新的RNase H抑制剂。在新合成的衍生物中,氧苯基吡咯基-吡唑类化合物显示出在低微摩尔/亚微摩尔范围内的抑制活性,其中化合物11b最有效。有趣的是,所有测试的化合物对RNase H与整合酶的选择性均显示高达2个数量级。在RNase H催化位点内的对接研究与定点诱变一起显示了关键的结构特征,这些结构特征可以赋予在RNase H内建立特定相互作用的能力。他们证明了我们的化合物能够与在携带抗药性变异的患者中分离的HIV-1亚种中高度保守的氨基酸相互作用。最后,新发现的吡唑羧酸衍生物相对于其相应的DKA具有良好的血清稳定性。
更新日期:2020-03-05
中文翻译:

吡咯基吡唑类化合物是HIV-1核糖核酸酶H逆转录酶功能的非二酮酸抑制剂。
由于二酮酸(DKA)链的生物责任,我们将先前报道的抗HIV-1吡咯基衍生物的这一元素转移到了非DKA支架上,获得了一系列吡咯基-吡唑羧酸作为新的RNase H抑制剂。在新合成的衍生物中,氧苯基吡咯基-吡唑类化合物显示出在低微摩尔/亚微摩尔范围内的抑制活性,其中化合物11b最有效。有趣的是,所有测试的化合物对RNase H与整合酶的选择性均显示高达2个数量级。在RNase H催化位点内的对接研究与定点诱变一起显示了关键的结构特征,这些结构特征可以赋予在RNase H内建立特定相互作用的能力。他们证明了我们的化合物能够与在携带抗药性变异的患者中分离的HIV-1亚种中高度保守的氨基酸相互作用。最后,新发现的吡唑羧酸衍生物相对于其相应的DKA具有良好的血清稳定性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号