当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecularly Engineered Nanobodies for Tunable Pharmacokinetics and Drug Delivery.
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-03-13 , DOI: 10.1021/acs.bioconjchem.0c00003 Patrick M Glassman 1 , Landis R Walsh 1 , Carlos H Villa 1 , Oscar A Marcos-Contreras 1 , Elizabeth D Hood 1 , Vladimir R Muzykantov 1 , Colin F Greineder 1, 2
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-03-13 , DOI: 10.1021/acs.bioconjchem.0c00003 Patrick M Glassman 1 , Landis R Walsh 1 , Carlos H Villa 1 , Oscar A Marcos-Contreras 1 , Elizabeth D Hood 1 , Vladimir R Muzykantov 1 , Colin F Greineder 1, 2
Affiliation
|
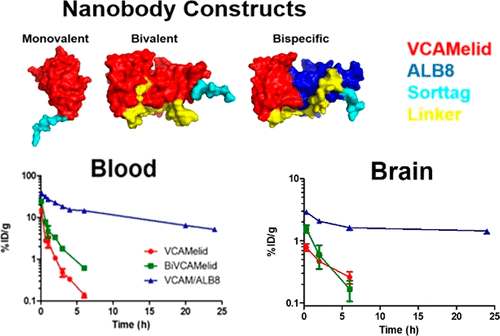
The use of single-domain antibody fragments, or nanobodies, has gained popularity in recent years as an alternative to traditional monoclonal antibody-based approaches. Relatively little is known, however, about the utility of nanobodies as targeting agents for delivery of therapeutic cargoes, particularly to vascular epitopes or in the setting of acute inflammatory conditions. We used a nanobody (VCAMelid) directed against mouse vascular cell adhesion molecule 1 (VCAM-1) and techniques for site-specific radiolabeling and bioconjugation to measure targeting to sites of constitutive and inducible antigen expression and investigate the impact of various characteristics (affinity, valence, circulation time) on nanobody biodistribution and pharmacokinetics. Engineering of VCAMelid for bivalent binding (BiVCAMelid) increased affinity by an order of magnitude and provided 2.8- and 3.6-fold enhancements in splenic and brain targeting in naive mice, with a further 2.6-fold increase in brain uptake in the setting of focal CNS inflammation. In contrast, introduction of an albumin-binding arm (VCAM/ALB8) did not affect binding affinity, but its prolonged circulation time resulted in 3.5-fold and 17.4-fold increases in splenic and brain uptake at 20 min post-dose and remarkable 40-, 25-, and 15-fold enhancements in overall exposure of blood, spleen, and brain, respectively, relative to both VCAMelid and BiVCAMelid. Both therapeutic protein (superoxide dismutase, SOD-1) and nanocarrier (liposome) delivery were enhanced by conjugation to VCAM-1 targeted nanobodies. The bispecific VCAM/ALB8 maintained its superiority over VCAMelid in enhancing both circulation time and organ targeting of SOD-1, but its advantages were largely blunted by conjugation to liposomes.
中文翻译:

用于可调药代动力学和药物递送的分子工程纳米抗体。
近年来,单域抗体片段或纳米抗体的使用已成为传统单克隆抗体方法的替代品。然而,关于纳米抗体作为靶向剂用于递送治疗货物的效用,尤其是血管表位或在急性炎症条件下的效用,我们知之甚少。我们使用针对小鼠血管细胞粘附分子 1 (VCAM-1) 的纳米抗体 (VCAMelid) 和位点特异性放射性标记和生物偶联技术来测量对组成型和诱导型抗原表达位点的靶向性,并研究各种特征(亲和力、价,循环时间)对纳米体生物分布和药代动力学的影响。用于二价结合的 VCAMelid 工程 (BiVCAMelid) 将亲和力提高了一个数量级,并在幼稚小鼠的脾脏和脑靶向方面提供了 2.8 倍和 3.6 倍的增强,在局灶性 CNS 环境中脑摄取进一步增加了 2.6 倍炎。相比之下,白蛋白结合臂 (VCAM/ALB8) 的引入不影响结合亲和力,但其延长的循环时间导致服药后 20 分钟脾和脑摄取增加 3.5 倍和 17.4 倍,显着增加 40 - 相对于 VCAMelid 和 BiVCAMelid,血液、脾脏和大脑的总体暴露分别提高了 25 倍和 15 倍。治疗性蛋白质(超氧化物歧化酶,SOD-1)和纳米载体(脂质体)的递送均通过与 VCAM-1 靶向纳米抗体的结合得到增强。
更新日期:2020-04-23
中文翻译:

用于可调药代动力学和药物递送的分子工程纳米抗体。
近年来,单域抗体片段或纳米抗体的使用已成为传统单克隆抗体方法的替代品。然而,关于纳米抗体作为靶向剂用于递送治疗货物的效用,尤其是血管表位或在急性炎症条件下的效用,我们知之甚少。我们使用针对小鼠血管细胞粘附分子 1 (VCAM-1) 的纳米抗体 (VCAMelid) 和位点特异性放射性标记和生物偶联技术来测量对组成型和诱导型抗原表达位点的靶向性,并研究各种特征(亲和力、价,循环时间)对纳米体生物分布和药代动力学的影响。用于二价结合的 VCAMelid 工程 (BiVCAMelid) 将亲和力提高了一个数量级,并在幼稚小鼠的脾脏和脑靶向方面提供了 2.8 倍和 3.6 倍的增强,在局灶性 CNS 环境中脑摄取进一步增加了 2.6 倍炎。相比之下,白蛋白结合臂 (VCAM/ALB8) 的引入不影响结合亲和力,但其延长的循环时间导致服药后 20 分钟脾和脑摄取增加 3.5 倍和 17.4 倍,显着增加 40 - 相对于 VCAMelid 和 BiVCAMelid,血液、脾脏和大脑的总体暴露分别提高了 25 倍和 15 倍。治疗性蛋白质(超氧化物歧化酶,SOD-1)和纳米载体(脂质体)的递送均通过与 VCAM-1 靶向纳米抗体的结合得到增强。









































 京公网安备 11010802027423号
京公网安备 11010802027423号