当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Analysis of Cj1427, an Essential NAD-Dependent Dehydrogenase for the Biosynthesis of the Heptose Residues in the Capsular Polysaccharides of Campylobacter jejuni.
Biochemistry ( IF 2.9 ) Pub Date : 2020-03-23 , DOI: 10.1021/acs.biochem.0c00096 Jamison P Huddleston 1 , Thomas K Anderson 2 , Keelan D Spencer 2 , James B Thoden 2 , Frank M Raushel 1, 3 , Hazel M Holden 2
Biochemistry ( IF 2.9 ) Pub Date : 2020-03-23 , DOI: 10.1021/acs.biochem.0c00096 Jamison P Huddleston 1 , Thomas K Anderson 2 , Keelan D Spencer 2 , James B Thoden 2 , Frank M Raushel 1, 3 , Hazel M Holden 2
Affiliation
|
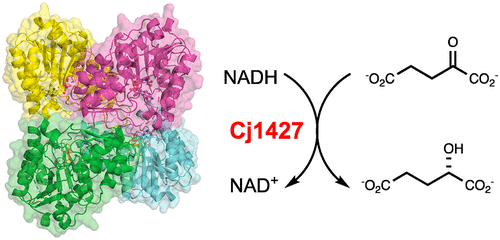
Many strains of Campylobacter jejuni display modified heptose residues in their capsular polysaccharides (CPS). The precursor heptose was previously shown to be GDP-d-glycero-α-d-manno-heptose, from which a variety of modifications of the sugar moiety have been observed. These modifications include the generation of 6-deoxy derivatives and alterations of the stereochemistry at C3–C6. Previous work has focused on the enzymes responsible for the generation of the 6-deoxy derivatives and those involved in altering the stereochemistry at C3 and C5. However, the generation of the 6-hydroxyl heptose residues remains uncertain due to the lack of a specific enzyme to catalyze the initial oxidation at C4 of GDP-d-glycero-α-d-manno-heptose. Here we reexamine the previously reported role of Cj1427, a dehydrogenase found in C. jejuni NTCC 11168 (HS:2). We show that Cj1427 is co-purified with bound NADH, thus hindering catalysis of oxidation reactions. However, addition of a co-substrate, α-ketoglutarate, converts the bound NADH to NAD+. In this form, Cj1427 catalyzes the oxidation of l-2-hydroxyglutarate back to α-ketoglutarate. The crystal structure of Cj1427 with bound GDP-d-glycero-α-d-manno-heptose shows that the NAD(H) cofactor is ideally positioned to catalyze the oxidation at C4 of the sugar substrate. Additionally, the overall fold of the Cj1427 subunit places it into the well-defined short-chain dehydrogenase/reductase superfamily. The observed quaternary structure of the tetrameric enzyme, however, is highly unusual for members of this superfamily.
中文翻译:

空肠弯曲杆菌荚膜多糖中庚糖残基生物合成必需的NAD依赖性脱氢酶Cj1427的结构分析。
空肠弯曲杆菌的许多菌株在其荚膜多糖(CPS)中显示修饰的庚糖残基。该前体庚糖先前显示为GDP- d -甘油基-α- d -甘露-heptose,从已经观察到各种糖部分的修饰。这些修饰包括6-脱氧衍生物的产生和C3-C6立体化学的改变。以前的工作集中在负责产生6-脱氧衍生物的酶以及涉及改变C3和C5立体化学的酶。但是,由于缺乏一种特定的酶来催化GDP-C4处的初始氧化,因此6-羟基庚糖残基的产生仍然不确定。d -甘油-α- d -甘露-heptose。在这里,我们重新检查了先前报道的空肠弯曲杆菌NTCC 11168(HS:2)中发现的脱氢酶Cj1427的作用。我们显示Cj1427是与结合的NADH共纯化的,因此阻碍了氧化反应的催化。但是,添加共底物α-酮戊二酸酯可将结合的NADH转化为NAD +。在这种形式中,Cj1427催化的氧化升-2-羟基回α酮戊二酸。Cj1427与晶体结构结合GDP- d -甘油基-α- d -甘露-庚糖表明NAD(H)辅助因子的位置理想,可催化糖底物C4处的氧化。此外,Cj1427亚基的整体折叠将其放入定义明确的短链脱氢酶/还原酶超家族。然而,观察到的四聚体酶的四级结构对于该超家族成员是非常不寻常的。
更新日期:2020-03-24
中文翻译:

空肠弯曲杆菌荚膜多糖中庚糖残基生物合成必需的NAD依赖性脱氢酶Cj1427的结构分析。
空肠弯曲杆菌的许多菌株在其荚膜多糖(CPS)中显示修饰的庚糖残基。该前体庚糖先前显示为GDP- d -甘油基-α- d -甘露-heptose,从已经观察到各种糖部分的修饰。这些修饰包括6-脱氧衍生物的产生和C3-C6立体化学的改变。以前的工作集中在负责产生6-脱氧衍生物的酶以及涉及改变C3和C5立体化学的酶。但是,由于缺乏一种特定的酶来催化GDP-C4处的初始氧化,因此6-羟基庚糖残基的产生仍然不确定。d -甘油-α- d -甘露-heptose。在这里,我们重新检查了先前报道的空肠弯曲杆菌NTCC 11168(HS:2)中发现的脱氢酶Cj1427的作用。我们显示Cj1427是与结合的NADH共纯化的,因此阻碍了氧化反应的催化。但是,添加共底物α-酮戊二酸酯可将结合的NADH转化为NAD +。在这种形式中,Cj1427催化的氧化升-2-羟基回α酮戊二酸。Cj1427与晶体结构结合GDP- d -甘油基-α- d -甘露-庚糖表明NAD(H)辅助因子的位置理想,可催化糖底物C4处的氧化。此外,Cj1427亚基的整体折叠将其放入定义明确的短链脱氢酶/还原酶超家族。然而,观察到的四聚体酶的四级结构对于该超家族成员是非常不寻常的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号