当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How Does Addition of Lithium Salt Influence the Structure and Dynamics of Choline Chloride-Based Deep Eutectic Solvents?
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.jpcb.9b11947 Sahadev Barik 1 , Manjari Chakraborty 1 , Moloy Sarkar 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.jpcb.9b11947 Sahadev Barik 1 , Manjari Chakraborty 1 , Moloy Sarkar 1
Affiliation
|
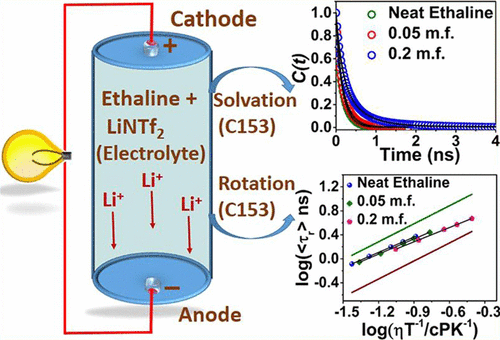
In recent times, deep eutectic solvents (DESs) have emerged as an environment-friendly alternative to both common organic solvents and ionic liquids (ILs). The present study has been undertaken with an objective to understand the intermolecular interaction, structural organization, and dynamics of two DES systems in the absence and presence of lithium salt so that the potential of these mixtures in electrochemical application is realized. For this purpose, the steady-state, time-resolved fluorescence, electron paramagnetic resonance (EPR), and nuclear magnetic resonance (NMR) behavior of two DESs (ethaline and glyceline) and their mixture with lithium bis(trifluoromethylsulfonyl) imide (LiNTf2) has been investigated. Measurements of polarity through EPR technique have revealed that the polarities of DESs are close to aliphatic polyhydroxy alcohol and the polarities of the medium increase with the increase in lithium salt concentration. Studies on solvation dynamics have indicated that there is an increase in average solvation time with the increase in lithium salt concentration. Investigation of rotational dynamics of some selected fluorophore in these media has shown that addition of lithium salt significantly alters the nano/microstructural organization of both DESs. Further, measurements of the self-diffusion coefficient through NMR have also supported the perturbation of the nanostructural organization of the solvent systems by addition of lithium salts. Essentially, all of these investigations have suggested that addition of lithium salt significantly alters the microscopic behavior of DESs. The outcome of this study is expected to be helpful in realizing the potential of these media for various electrochemical applications including application in lithium-ion battery.
中文翻译:

锂盐的添加如何影响基于胆碱氯化物的深共晶溶剂的结构和动力学?
近年来,深共晶溶剂(DES)逐渐成为普通有机溶剂和离子液体(IL)的环保替代品。进行本研究的目的是了解在不存在和存在锂盐的情况下两个DES系统的分子间相互作用,结构组织和动力学,从而实现这些混合物在电化学应用中的潜力。为此,两个DES(乙醇胺和甘油)及其与双(三氟甲基磺酰基)酰亚胺锂(LiNTf 2)的混合物的稳态,时间分辨荧光,电子顺磁共振(EPR)和核磁共振(NMR)行为)已被调查。通过EPR技术进行的极性测量表明,DES的极性接近于脂肪族多羟基醇,并且介质的极性随锂盐浓度的增加而增加。溶剂化动力学研究表明,平均溶剂化时间随锂盐浓度的增加而增加。在这些介质中一些选定的荧光团的旋转动力学研究表明,添加锂盐会显着改变两种DES的纳米/微观结构。此外,通过NMR对自扩散系数的测量还支持了通过添加锂盐对溶剂体系的纳米结构组织的扰动。本质上,所有这些研究表明,添加锂盐会显着改变DES的微观行为。预期这项研究的结果将有助于实现这些介质在各种电化学应用中的潜力,包括在锂离子电池中的应用。
更新日期:2020-03-31
中文翻译:

锂盐的添加如何影响基于胆碱氯化物的深共晶溶剂的结构和动力学?
近年来,深共晶溶剂(DES)逐渐成为普通有机溶剂和离子液体(IL)的环保替代品。进行本研究的目的是了解在不存在和存在锂盐的情况下两个DES系统的分子间相互作用,结构组织和动力学,从而实现这些混合物在电化学应用中的潜力。为此,两个DES(乙醇胺和甘油)及其与双(三氟甲基磺酰基)酰亚胺锂(LiNTf 2)的混合物的稳态,时间分辨荧光,电子顺磁共振(EPR)和核磁共振(NMR)行为)已被调查。通过EPR技术进行的极性测量表明,DES的极性接近于脂肪族多羟基醇,并且介质的极性随锂盐浓度的增加而增加。溶剂化动力学研究表明,平均溶剂化时间随锂盐浓度的增加而增加。在这些介质中一些选定的荧光团的旋转动力学研究表明,添加锂盐会显着改变两种DES的纳米/微观结构。此外,通过NMR对自扩散系数的测量还支持了通过添加锂盐对溶剂体系的纳米结构组织的扰动。本质上,所有这些研究表明,添加锂盐会显着改变DES的微观行为。预期这项研究的结果将有助于实现这些介质在各种电化学应用中的潜力,包括在锂离子电池中的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号