当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactions of Transition-Metal Carbyne Cations with Ethylene in the Gas Phase
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-03-23 , DOI: 10.1021/acs.jpca.0c00371 Xiao-Nan Wu 1 , Zizhuang Liu 1 , Hechen Wu 1 , Di Zhang 1 , Wei Li 1 , Zejian Huang 1 , Guanjun Wang 1 , Fuxing Xu 1 , Chuan-fan Ding 1 , Mingfei Zhou 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-03-23 , DOI: 10.1021/acs.jpca.0c00371 Xiao-Nan Wu 1 , Zizhuang Liu 1 , Hechen Wu 1 , Di Zhang 1 , Wei Li 1 , Zejian Huang 1 , Guanjun Wang 1 , Fuxing Xu 1 , Chuan-fan Ding 1 , Mingfei Zhou 1
Affiliation
|
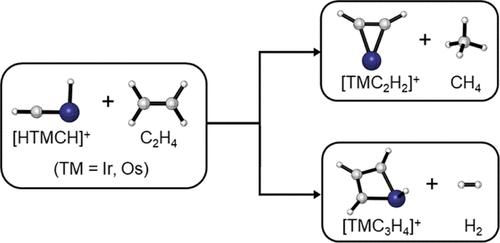
The reactions of iridium- and osmium-carbyne hydride cations [HIrCH]+ and [HOsCH]+ with ethylene have been studied using mass spectrometry with isotopic-labeling in the gas phase. The carbyne reactivity is compared with that of the rhodium, cobalt, and iron analogues [TMCH2]+ (TM = Fe, Co, and Rh), which were determined to have the carbene structures. Besides the cycloaddition/dehydrogenation reaction in forming the [TMC3H4]+ + H2 (TM = Ir and Os) products, a second reaction pathway producing the [TMC2H2]+ ion and CH4 via triple hydrogen atom transfer reactions to the carbyne carbon is observed to be the major channel. The latter channel is not observed in the rhodium, cobalt, and iron carbene cation reactions. Quantum-chemical calculations indicate that the distinct reactivity is not due to different initial structures of the reactants. Both reaction channels are predicted to be thermodynamically exothermic and kinetically facile for the carbyne cations, and the reactions proceed with the initial formation of a carbene intermediate via hydride–carbyne coupling. The latter channel is also exothermic but kinetically unfavorable for the rhodium, cobalt, and iron carbene cations.
中文翻译:

气相中过渡金属卡宾阳离子与乙烯的反应
使用质谱和同位素标记技术在气相中研究了铱和carb-碳炔氢化物阳离子[HIrCH] +和[HOsCH] +与乙烯的反应。将卡宾反应性与确定具有卡宾结构的铑,钴和铁类似物[TMCH 2 ] +(TM = Fe,Co和Rh)进行比较。除了形成[TMC 3 H 4 ] + + H 2(TM = Ir和Os)产物时的环加成/脱氢反应外,还有第二种产生[TMC 2 H 2 ] +离子和CH 4的反应路径。通过三氢原子转移反应到碳炔是主要途径。在铑,钴和铁卡宾阳离子反应中未观察到后一个通道。量子化学计算表明,不同的反应性不是由于反应物的不同初始结构。预测这两个反应通道对于卡宾阳离子都是热力学放热的,并且在动力学上很容易,并且反应通过氢化物-卡宾偶联初始形成卡宾中间体而继续进行。后者的通道也是放热的,但对于铑,钴和铁卡宾阳离子在动力学上不利。
更新日期:2020-03-24
中文翻译:

气相中过渡金属卡宾阳离子与乙烯的反应
使用质谱和同位素标记技术在气相中研究了铱和carb-碳炔氢化物阳离子[HIrCH] +和[HOsCH] +与乙烯的反应。将卡宾反应性与确定具有卡宾结构的铑,钴和铁类似物[TMCH 2 ] +(TM = Fe,Co和Rh)进行比较。除了形成[TMC 3 H 4 ] + + H 2(TM = Ir和Os)产物时的环加成/脱氢反应外,还有第二种产生[TMC 2 H 2 ] +离子和CH 4的反应路径。通过三氢原子转移反应到碳炔是主要途径。在铑,钴和铁卡宾阳离子反应中未观察到后一个通道。量子化学计算表明,不同的反应性不是由于反应物的不同初始结构。预测这两个反应通道对于卡宾阳离子都是热力学放热的,并且在动力学上很容易,并且反应通过氢化物-卡宾偶联初始形成卡宾中间体而继续进行。后者的通道也是放热的,但对于铑,钴和铁卡宾阳离子在动力学上不利。









































 京公网安备 11010802027423号
京公网安备 11010802027423号