当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tumor-Specific Endogenous FeII-Activated, MRI-Guided Self-Targeting Gadolinium-Coordinated Theranostic Nanoplatforms for Amplification of ROS and Enhanced Chemodynamic Chemotherapy
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-03-18 , DOI: 10.1021/acsami.0c00970 Zhongxiong Fan 1 , Beili Jiang 1 , Qixin Zhu 2 , Sijin Xiang 3 , Li Tu 1 , Yifan Yang 1 , Qingliang Zhao 4 , Doudou Huang 4 , Jian Han 5 , Guanghao Su 6 , Dongtao Ge 1 , Zhenqing Hou 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-03-18 , DOI: 10.1021/acsami.0c00970 Zhongxiong Fan 1 , Beili Jiang 1 , Qixin Zhu 2 , Sijin Xiang 3 , Li Tu 1 , Yifan Yang 1 , Qingliang Zhao 4 , Doudou Huang 4 , Jian Han 5 , Guanghao Su 6 , Dongtao Ge 1 , Zhenqing Hou 1
Affiliation
|
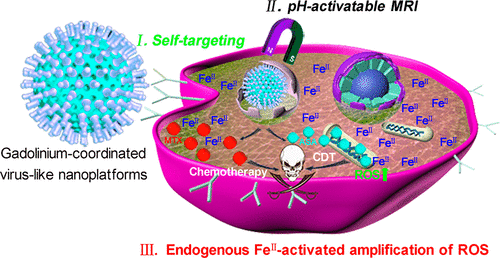
Low drug payload and lack of tumor-targeting for chemodynamic therapy (CDT) result in an insufficient reactive oxygen species (ROS) generation, which seriously hinders its further clinical application. Therefore, how to improve the drug payload and tumor targeting for amplification of ROS and combine it with chemotherapy has been a huge challenge in CDT. Herein, methotrexate (MTX), gadolinium (Gd), and artesunate (ASA) were used as theranostic building blocks to be coordinately assembled into tumor-specific endogenous FeII-activated and magnetic resonance imaging (MRI)-guided self-targeting carrier-free nanoplatforms (NPs) for amplification of ROS and enhanced chemodynamic chemotherapy. The obtained ASA-MTX-GdIII NPs exhibited extremely high drug payload (∼96 wt %), excellent physiological stability, long circulating ability (half-time: ∼12 h), and outstanding tumor accumulation. Moreover, ASA-MTX-GdIII NPs could be specifically uptaken by tumor cells via folate (FA) receptors and subsequently be disassembled via lysosomal acidity-induced coordination breakage, resulting in drug burst release. Most strikingly, the produced ASA could be catalyzed by tumor-specific overexpressed endogenous FeII ions to generate sufficient ROS for enhancing the main chemodynamic efficacy, which could exert a synergistic effect with the assistant chemotherapy of MTX. Interestingly, ASA-MTX-GdIII NPs caused a lower ROS generation and toxicity on normal cell lines that seldom expressed endogenous FeII ions. Under MRI guidance with assistance of self-targeting, significantly superior synergistic tumor therapy was performed on FA receptor-overexpressed tumor-bearing mice with a higher ROS generation and an almost complete elimination of tumor. This work highlights ASA-MTX-GdIII NPs as an efficient chemodynamic-chemotherapeutic agent for MRI imaging and tumor theranostics.
中文翻译:

特定于肿瘤的内源性Fe II激活,MRI引导的自靶向Ga协同的治疗性纳米平台,用于扩增ROS和增强化学动力化学疗法。
低药物有效载荷和缺乏针对肿瘤的化学动力学疗法(CDT)导致活性氧(ROS)生成不足,严重阻碍了其进一步的临床应用。因此,如何改善药物有效载荷和靶向性以扩增ROS并将其与化学疗法相结合一直是CDT面临的巨大挑战。在此,将甲氨蝶呤(MTX),((Gd)和青蒿琥酯(ASA)用作治疗学构建基块,以协调组装到肿瘤特异性内源性Fe II激活和磁共振成像(MRI)引导的自靶向载体-游离纳米平台(NPs)用于扩增ROS和增强化学动力化疗。获得的ASA-MTX-Gd IIINPs表现出极高的药物有效载荷(〜96 wt%),出色的生理稳定性,长循环能力(半衰期:〜12 h)和出色的肿瘤蓄积性。此外,ASA-MTX-Gd III NPs可以通过叶酸(FA)受体被肿瘤细胞特异性摄取,随后通过溶酶体酸性诱导的配位破坏而分解,从而导致药物爆发释放。最为显着的是,所产生的ASA可以被肿瘤特异性过表达的内源性Fe II离子催化产生足够的ROS来增强主要的化学动力学功效,从而可以与MTX的辅助化疗产生协同作用。有趣的是,ASA-MTX-Gd IIINPs在很少表达内源性Fe II离子的正常细胞系上引起较低的ROS生成和毒性。在MRI指导下,在自我靶向的辅助下,对具有较高ROS生成和几乎完全消除肿瘤的FA受体过度表达的荷瘤小鼠进行了明显优越的协同肿瘤治疗。这项工作强调了ASA-MTX-Gd III NPs是一种用于MRI成像和肿瘤治疗的有效化学动力化学治疗剂。
更新日期:2020-03-19
中文翻译:

特定于肿瘤的内源性Fe II激活,MRI引导的自靶向Ga协同的治疗性纳米平台,用于扩增ROS和增强化学动力化学疗法。
低药物有效载荷和缺乏针对肿瘤的化学动力学疗法(CDT)导致活性氧(ROS)生成不足,严重阻碍了其进一步的临床应用。因此,如何改善药物有效载荷和靶向性以扩增ROS并将其与化学疗法相结合一直是CDT面临的巨大挑战。在此,将甲氨蝶呤(MTX),((Gd)和青蒿琥酯(ASA)用作治疗学构建基块,以协调组装到肿瘤特异性内源性Fe II激活和磁共振成像(MRI)引导的自靶向载体-游离纳米平台(NPs)用于扩增ROS和增强化学动力化疗。获得的ASA-MTX-Gd IIINPs表现出极高的药物有效载荷(〜96 wt%),出色的生理稳定性,长循环能力(半衰期:〜12 h)和出色的肿瘤蓄积性。此外,ASA-MTX-Gd III NPs可以通过叶酸(FA)受体被肿瘤细胞特异性摄取,随后通过溶酶体酸性诱导的配位破坏而分解,从而导致药物爆发释放。最为显着的是,所产生的ASA可以被肿瘤特异性过表达的内源性Fe II离子催化产生足够的ROS来增强主要的化学动力学功效,从而可以与MTX的辅助化疗产生协同作用。有趣的是,ASA-MTX-Gd IIINPs在很少表达内源性Fe II离子的正常细胞系上引起较低的ROS生成和毒性。在MRI指导下,在自我靶向的辅助下,对具有较高ROS生成和几乎完全消除肿瘤的FA受体过度表达的荷瘤小鼠进行了明显优越的协同肿瘤治疗。这项工作强调了ASA-MTX-Gd III NPs是一种用于MRI成像和肿瘤治疗的有效化学动力化学治疗剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号