JAMA Oncology ( IF 22.5 ) Pub Date : 2020-05-01 , DOI: 10.1001/jamaoncol.2019.6828 Isamu Okamoto 1 , Hiroshi Nokihara 2 , Shogo Nomura 3 , Seiji Niho 4 , Shunichi Sugawara 5 , Hidehito Horinouchi 6 , Koichi Azuma 7 , Yasuto Yoneshima 1 , Haruyasu Murakami 8 , Yukio Hosomi 9 , Shinji Atagi 10 , Tomohiro Ozaki 11 , Atsushi Horiike 12 , Yuka Fujita 13 , Hiroaki Okamoto 14 , Masahiko Ando 15 , Nobuyuki Yamamoto 16 , Yuichiro Ohe 6 , Kazuhiko Nakagawa 17
|
Importance Few clinical trials have been specifically designed for elderly patients with advanced non–small cell lung cancer (NSCLC), and the anticipated increase in the number of such patients has prompted a search for new treatment options that provide a greater palliative benefit.
Objective To determine whether treatment with carboplatin plus pemetrexed followed by pemetrexed maintenance is noninferior compared with docetaxel monotherapy with regard to overall survival (OS) for elderly patients with advanced nonsquamous NSCLC.
Design, Setting, and Participants This open-label, multicenter, noninferiority phase 3 randomized clinical trial was conducted at 79 institutions in Japan. Cytotoxic chemotherapy–naive patients with advanced nonsquamous NSCLC, an Eastern Cooperative Oncology Group performance status of 0 or 1, and age of 75 years or older were enrolled between August 2013 and February 2017. Data were analyzed from November 2018 to February 2019.
Interventions Patients were randomized to receive either docetaxel monotherapy (60 mg/m2) every 3 weeks or 4 cycles of carboplatin (area under the curve of 5) plus pemetrexed (500 mg/m2) administered every 3 weeks followed by maintenance therapy with the same dose of pemetrexed for 3 weeks.
Main Outcomes and Measures The primary end point was OS analyzed on an intention-to-treat basis with a noninferiority margin of 1.154 for the upper limit of the 95% CI of the hazard ratio (HR) estimated with a stratified Cox regression model.
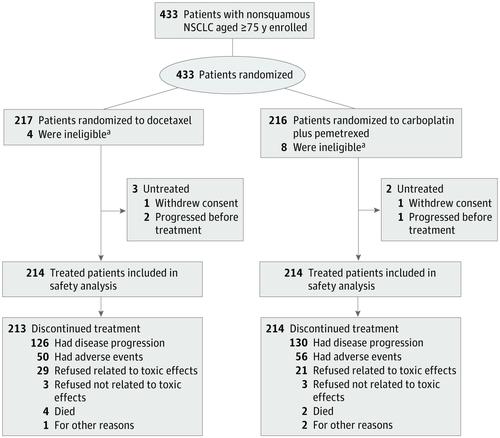
Results Of the 433 enrolled patients, 250 (57.7%) were male, and the median (range) age was 78 (75-88) years. The median OS was 15.5 months (95% CI, 13.6-18.4) in the docetaxel group (n = 217) and 18.7 months (95% CI, 16.0-21.9) in the carboplatin-pemetrexed group (n = 216), with a stratified HR for OS of 0.850 (95% CI, 0.684-1.056; P for noninferiority = .003). Progression-free survival was also longer in the carboplatin-pemetrexed group (unstratified HR, 0.739; 95% CI, 0.609-0.896). Compared with those in the docetaxel group, those in the carboplatin-pemetrexed had lower rates of leukopenia (60 of 214 [28.0%] vs 147 of 214 [68.7%]) and neutropenia (99 of 214 [46.3%] vs 184 of 214 [86.0%]) of grade 3 or 4 and of febrile neutropenia (9 of 214 [4.2%] vs 38 of 214 [17.8%]) and higher rates of thrombocytopenia (55 of 214 [25.7%] vs 3 of 214 [1.4%]) and anemia (63 of 214 [29.4%] vs 4 of 214 [1.9%]) of grade 3 or 4. Dose reductions were less frequent with carboplatin-pemetrexed.
Conclusion and Relevance Carboplatin-pemetrexed treatment followed by pemetrexed maintenance is a valid option for first-line treatment of elderly patients with advanced nonsquamous NSCLC.
Trial Registration University Hospital Medical Information Network Clinical Trials Registry Identifier: UMIN000011460
中文翻译:

晚期非鳞状非小细胞肺癌老年患者卡铂加培美曲塞加培美曲塞联合多西他赛单药维持治疗的比较:一项3期随机临床试验。
重要性 很少有专门针对老年晚期非小细胞肺癌(NSCLC)患者的临床试验,此类患者数量的预期增加促使人们寻求可提供更大姑息作用的新治疗方案。
目的 确定在晚期非鳞状NSCLC老年患者的总生存(OS)方面,与多西他赛单一疗法相比,卡铂联合培美曲塞联合培美曲塞维持治疗的疗效是否逊于多西他赛单药治疗。
设计,设置和参与者 这项开放性,多中心,非劣效性第3期随机临床试验在日本的79家机构中进行。在2013年8月至2017年2月之间,纳入了未经细胞毒性化疗的初发非鳞状NSCLC晚期患者,东部合作肿瘤小组的工作状态为0或1,年龄在75岁或75岁以上的患者。数据分析了2018年11月至2019年2月的数据。
干预措施 患者随机接受每3周一次多西他赛单药治疗(60 mg / m 2)或4个周期的卡铂(5点以下的面积)加上培美曲塞(500 mg / m 2)每3周一次,然后进行维持治疗相同剂量的培美曲塞3周。
主要结果和措施 主要终点是按意向性治疗进行OS分析,非劣质性边际值为1.154,这是通过分层Cox回归模型估算的危险比(HR)95%CI的上限。
结果 433例患者中,男性250例(57.7%),中位年龄范围为78岁(75-88)。多西紫杉醇组(n = 217)的中位OS为15.5个月(95%CI,13.6-18.4),卡铂培美曲塞组(n = 216)的中位OS为18.7个月(95%CI,16.0-21.9)。 OS的分层HR为0.850(95%CI,0.684-1.056; P非劣等= 0.003)。卡铂培美曲塞组的无进展生存期也更长(未分层HR,0.739; 95%CI,0.609-0.896)。与多西他赛组相比,卡铂-培美曲塞组的白细胞减少症(214例中的60例[28.0%]比214例中的147例[68.7%])和中性粒细胞减少症的发生率较低(214例中的99例[46.3%]与184例的184例3或4级和发热性中性粒细胞减少症[86.0%](214例9 [4.2%]对214例38 [17.8%])和血小板减少症的发生率较高(214例55 [25.7%]对214例3 [1.4] %和3级或4级贫血(214例中的63 [29.4%]比214例中的4例[1.9%])减少了卡铂培美曲塞治疗的剂量减少。
结论与相关性 卡铂培美曲塞联合培美曲塞维持治疗是老年晚期非鳞状非小细胞肺癌一线治疗的有效选择。
试验注册 大学医院医学信息网络临床试验注册标识:UMIN000011460











































 京公网安备 11010802027423号
京公网安备 11010802027423号