当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of indole based acetohydrazide analogs: Their in vitro and in silico thymidine phosphorylase studies.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-03-12 , DOI: 10.1016/j.bioorg.2020.103745 Muhammad Taha 1 , Ebaa Ahmed Jassim Aldhamin 2 , Noor Barak Almandil 1 , El Hassane Anouar 3 , Nizam Uddin 4 , Munther Alomari 5 , Fazal Rahim 6 , Bushra Adalat 6 , Mohamad Ibrahim 1 , Fasial Nawaz 7 , Naveed Iqbal 8 , Bandar Alghanem 9 , Abdulelah Altolayyan 9 , Khalid Mohammed Khan 10
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-03-12 , DOI: 10.1016/j.bioorg.2020.103745 Muhammad Taha 1 , Ebaa Ahmed Jassim Aldhamin 2 , Noor Barak Almandil 1 , El Hassane Anouar 3 , Nizam Uddin 4 , Munther Alomari 5 , Fazal Rahim 6 , Bushra Adalat 6 , Mohamad Ibrahim 1 , Fasial Nawaz 7 , Naveed Iqbal 8 , Bandar Alghanem 9 , Abdulelah Altolayyan 9 , Khalid Mohammed Khan 10
Affiliation
|
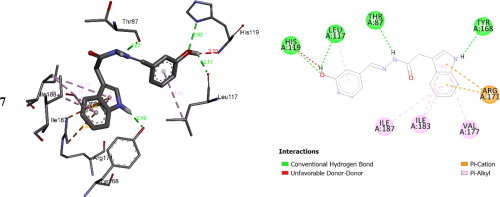
In this study, a series of indole based acetohydrazide derivatives (1-22) were synthesized and characterized by 13C NMR, 1H NMR and HREI-MS. The resulted derivatives were tested for thymidine phosphorylase inhibitory potential. These derivatives inhibited thymidine phosphorylase at different concentration ranging from 1.10 ± 0.10 to 41.10 ± 1.10 µM when compared with the standard 7-Deazaxanthine (IC50 value 38.68 ± 1.12 µM). The compound 8 having OH group at 2, 4 and 6 position was found the most potent among the series with IC50 1.10 ± 0.10 µM. The structure activity relationships (SAR) has been established for all compounds keeping in the view the role of substitution and the effect of functional group which significantly affect thymidine phosphorylase activity. The nature of binding interactions of the most potent compounds and active sites of the enzymes was confirmed through molecular docking study.
中文翻译:

吲哚基乙酰肼类似物的合成:他们的体外和计算机胸苷磷酸化酶研究。
在这项研究中,合成了一系列基于吲哚的乙酰肼衍生物(1-22),并通过13C NMR,1H NMR和HREI-MS对其进行了表征。测试所得衍生物的胸苷磷酸化酶抑制潜能。与标准7-脱氮黄嘌呤(IC50值38.68±1.12 µM)相比,这些衍生物抑制胸苷磷酸化酶的浓度范围为1.10±0.10至41.10±1.10 µM。发现在2、4和6位具有OH基的化合物8在IC50 1.10±0.10 µM的系列中最有效。考虑到取代的作用和显着影响胸苷磷酸化酶活性的官能团的作用,已经为所有化合物建立了结构活性关系(SAR)。
更新日期:2020-03-12
中文翻译:

吲哚基乙酰肼类似物的合成:他们的体外和计算机胸苷磷酸化酶研究。
在这项研究中,合成了一系列基于吲哚的乙酰肼衍生物(1-22),并通过13C NMR,1H NMR和HREI-MS对其进行了表征。测试所得衍生物的胸苷磷酸化酶抑制潜能。与标准7-脱氮黄嘌呤(IC50值38.68±1.12 µM)相比,这些衍生物抑制胸苷磷酸化酶的浓度范围为1.10±0.10至41.10±1.10 µM。发现在2、4和6位具有OH基的化合物8在IC50 1.10±0.10 µM的系列中最有效。考虑到取代的作用和显着影响胸苷磷酸化酶活性的官能团的作用,已经为所有化合物建立了结构活性关系(SAR)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号