Tetrahedron ( IF 2.1 ) Pub Date : 2020-03-12 , DOI: 10.1016/j.tet.2020.131127 Jyoti P. Mukherjee , Joyeeta Roy , Chyree S. Batton , Saroj Yadav , Douglas Wong , Carol M. Taylor
|
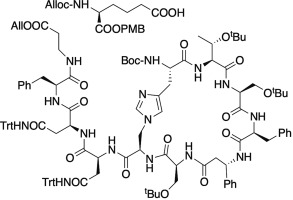
An orthogonally protected τ-histidinoalanine residue was synthesized via regioselective alkylation of optically pure Boc-l-His-OTCE (TCE = trichloroethyl) with a sulfamidate electrophile derived from Fmoc-d-Ser-OBn. The goal was the synthesis of a non-natural theonellamide, invoking readily accessible variants of the other 10 amino acids. Peptide fragments corresponding to the east and west rings of “theonellamide X″ were synthesized in solution. Each ring was formed independently, providing insights into protecting group limitations, side reactions, and the optimal order of events to approach the formation of the bicyclic system. Ultimately, an undecapeptide was prepared, with the eastern ring formed. The twelfth amino acid, an l-α-aminoadipic acid building block, was prepared and preliminary investigations into its attachment to the undecapeptide are reported.
中文翻译:

双环Theonellamide骨架的组装进展
的正交保护的τ-histidinoalanine残余物通过旋光纯的Boc-的区域选择性烷基化,合成升与亲电子从的Fmoc-衍生的磺酰胺酯-His-OTCE(TCE =三氯乙基)d -Ser-OBn的 目的是合成一种非天然的theonellamide,并调用其他10个氨基酸的易于获得的变体。在溶液中合成了与“ theonellamide X”的东环和西环相对应的肽片段。每个环都是独立形成的,可深入了解保护基团的限制,副反应以及接近双环系统形成的最佳事件顺序。最终,制备了十一肽,形成了东环。第十二氨基酸,升制备了-α-氨基己二酸构件,并报道了其与十一肽的连接的初步研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号