当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Screening of Transition Metal–Phthalocyanines for the Electrochemical Reduction of Carbon Dioxide
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-03-25 , DOI: 10.1021/acs.jpcc.9b10815 Gurpreet Kour 1, 2 , Xin Mao 1, 2 , Aijun Du 1, 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-03-25 , DOI: 10.1021/acs.jpcc.9b10815 Gurpreet Kour 1, 2 , Xin Mao 1, 2 , Aijun Du 1, 2
Affiliation
|
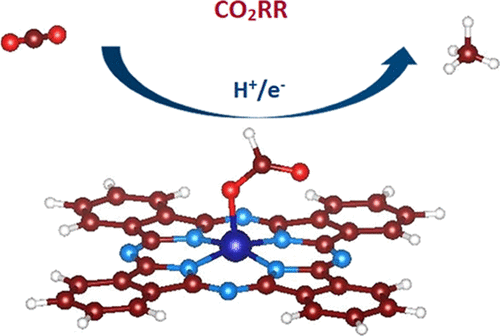
Molecular complexes containing low-cost transition-metal (TM) centers have been extensively studied for the electrochemical reduction of carbon dioxide. Of all the molecular catalysts reported so far, only a few of them are selective for CO2 reduction, and moreover, these catalysts mainly produce carbon monoxide or formic acid. However, molecular catalysts generating highly reduced products such as hydrocarbons are very rare. Herein, we explore the electrocatalytic activity of TM–Phthalocyanine (TM-Pc) by placing different transition metals into the vacant N4 cavity toward the reduction of CO2. By using first-principles calculations, we demonstrate that among all the 3d transition metals used, Chromium−Phthalocyanine (Cr-Pc)–Pc shows excellent performance for converting CO2 to methane with a limiting potential of −0.34 V. In comparison, the limiting potentials for the CO2 reduction reaction (CO2RR) to CH4 for the best catalyst considered so far such as Cu(111) and Cu(211) are −0.93 V and −0.74 V, respectively. Chromium, being a non-noble metal, presents as a promising TM for catalyzing CO2RR. Co-Pc however converts CO2 to methanol with a limiting potential of −0.69 V. This report shows that Pc with different TMs can provide an effective pathway for tuning the catalytic performance of electrocatalysts, which could help in the design of molecular catalysts in the future that will expectantly soon emerge at an industrial scale.
中文翻译:

用于二氧化碳电化学还原的过渡金属酞菁的计算筛选
包含低成本过渡金属(TM)中心的分子配合物已被广泛研究用于二氧化碳的电化学还原。迄今为止报道的所有分子催化剂中,只有少数对CO 2还原具有选择性,而且这些催化剂主要产生一氧化碳或甲酸。然而,产生高度还原的产物例如烃的分子催化剂是非常罕见的。在这里,我们通过将不同的过渡金属放入空的N 4空穴中以减少CO 2来探索TM-酞菁(TM-Pc)的电催化活性。。通过使用第一性原理计算,我们证明了在所使用的所有3d过渡金属中,铬-酞菁(Cr-Pc)-Pc在将CO 2转化为甲烷的极限电势为-0.34 V方面表现出出色的性能。对于迄今为止考虑的最佳催化剂,例如Cu(111)和Cu(211),CO 2还原反应(CO 2 RR)生成CH 4的极限电位分别为-0.93 V和-0.74V。铬是一种非贵金属,是一种有前景的可催化CO 2 RR的TM 。Co-Pc可转换CO 2 到极限电位为-0.69 V的甲醇中。该报告显示,具有不同TM的Pc可以为调节电催化剂的催化性能提供有效的途径,这可能有助于将来设计分子催化剂,预计不久后会出现。工业规模。
更新日期:2020-03-26
中文翻译:

用于二氧化碳电化学还原的过渡金属酞菁的计算筛选
包含低成本过渡金属(TM)中心的分子配合物已被广泛研究用于二氧化碳的电化学还原。迄今为止报道的所有分子催化剂中,只有少数对CO 2还原具有选择性,而且这些催化剂主要产生一氧化碳或甲酸。然而,产生高度还原的产物例如烃的分子催化剂是非常罕见的。在这里,我们通过将不同的过渡金属放入空的N 4空穴中以减少CO 2来探索TM-酞菁(TM-Pc)的电催化活性。。通过使用第一性原理计算,我们证明了在所使用的所有3d过渡金属中,铬-酞菁(Cr-Pc)-Pc在将CO 2转化为甲烷的极限电势为-0.34 V方面表现出出色的性能。对于迄今为止考虑的最佳催化剂,例如Cu(111)和Cu(211),CO 2还原反应(CO 2 RR)生成CH 4的极限电位分别为-0.93 V和-0.74V。铬是一种非贵金属,是一种有前景的可催化CO 2 RR的TM 。Co-Pc可转换CO 2 到极限电位为-0.69 V的甲醇中。该报告显示,具有不同TM的Pc可以为调节电催化剂的催化性能提供有效的途径,这可能有助于将来设计分子催化剂,预计不久后会出现。工业规模。











































 京公网安备 11010802027423号
京公网安备 11010802027423号