当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel, Dual Target-Directed Annelated Xanthine Derivatives Acting on Adenosine Receptors and Monoamine Oxidase B.
ChemMedChem ( IF 3.6 ) Pub Date : 2020-04-06 , DOI: 10.1002/cmdc.201900717 Kamil J Kuder 1 , Michał Załuski 1 , Jakub Schabikowski 1 , Gniewomir Latacz 1 , Agnieszka Olejarz-Maciej 1 , Piotr Jaśko 1 , Agata Doroz-Płonka 1 , Andreas Brockmann 2 , Christa E Müller 2 , Katarzyna Kieć-Kononowicz 1
ChemMedChem ( IF 3.6 ) Pub Date : 2020-04-06 , DOI: 10.1002/cmdc.201900717 Kamil J Kuder 1 , Michał Załuski 1 , Jakub Schabikowski 1 , Gniewomir Latacz 1 , Agnieszka Olejarz-Maciej 1 , Piotr Jaśko 1 , Agata Doroz-Płonka 1 , Andreas Brockmann 2 , Christa E Müller 2 , Katarzyna Kieć-Kononowicz 1
Affiliation
|
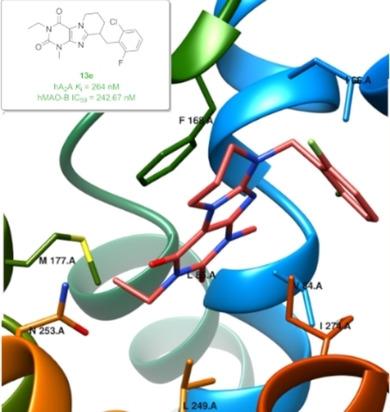
Annelated purinedione derivatives have been shown to act as possible multiple-target ligands, addressing adenosine receptors and monoaminooxidases. In this study, based on our previous results, novel annelated pyrimido- and diazepino[2,1-f]purinedione derivatives were designed as dual-target-directed ligands combining A2A adenosine receptor (AR) antagonistic activity with blocking monoamine oxidase B. A library of 19 novel compounds was synthesized and biologically evaluated in radioligand binding studies at AR subtypes and for their ability to inhibit MAO-B. This allowed 9-(2-chloro-6-fluorobenzyl)-3-ethyl-1-methyl-6,7,8,9-tetrahydropyrimido[2,1-f]purine-2,4(1H,3H)-dione (13 e; Ki human A2A AR: 264 nM and IC50 human MAO-B: 243 nM) to be identified as the most potent dual-acting ligand from this series. ADMET parameters were estimated in vitro, and analysis of the structure-activity relationships was complemented by molecular-docking studies based on previously published X-ray structures of the protein targets. Such dual-acting ligands, by selectively blocking A2A AR, accompanied by the inhibition of dopamine metabolizing enzyme MAO-B, might provide symptomatic and neuroprotective effects in, among others, the treatment of Parkinson disease.
中文翻译:

新型,双靶标定向的黄嘌呤衍生物作用于腺苷受体和单胺氧化酶B。
已显示出退火的嘌呤二酮衍生物可作为可能的多靶点配体,解决腺苷受体和单氨基氧化酶。在这项研究中,基于我们先前的结果,将新型的退火的嘧啶基和二氮杂环庚烷[2,1-f]嘌呤二酮衍生物设计为结合A2A腺苷受体(AR)拮抗活性和封闭单胺氧化酶B的双靶标配体。合成了19种新化合物的文库,并通过放射性配体结合研究对AR亚型及其抑制MAO-B的能力进行了生物学评估。这允许9-(2-氯-6-氟苄基)-3-乙基-1-甲基-6,7,8,9-四氢嘧啶基[2,1-f]嘌呤-2,4(1H,3H)-二酮(13 e; Ki人A2A AR:264 nM和IC50人MAO-B:243 nM)被确定为该系列中最有效的双作用配体。ADMET参数是在体外估算的,基于先前发表的蛋白质靶标X射线结构的分子对接研究,对结构-活性关系进行了分析和补充。通过选择性地阻断A2A AR,同时抑制多巴胺代谢酶MAO-B,这种双作用配体可能在帕金森病治疗中提供症状和神经保护作用。
更新日期:2020-04-06
中文翻译:

新型,双靶标定向的黄嘌呤衍生物作用于腺苷受体和单胺氧化酶B。
已显示出退火的嘌呤二酮衍生物可作为可能的多靶点配体,解决腺苷受体和单氨基氧化酶。在这项研究中,基于我们先前的结果,将新型的退火的嘧啶基和二氮杂环庚烷[2,1-f]嘌呤二酮衍生物设计为结合A2A腺苷受体(AR)拮抗活性和封闭单胺氧化酶B的双靶标配体。合成了19种新化合物的文库,并通过放射性配体结合研究对AR亚型及其抑制MAO-B的能力进行了生物学评估。这允许9-(2-氯-6-氟苄基)-3-乙基-1-甲基-6,7,8,9-四氢嘧啶基[2,1-f]嘌呤-2,4(1H,3H)-二酮(13 e; Ki人A2A AR:264 nM和IC50人MAO-B:243 nM)被确定为该系列中最有效的双作用配体。ADMET参数是在体外估算的,基于先前发表的蛋白质靶标X射线结构的分子对接研究,对结构-活性关系进行了分析和补充。通过选择性地阻断A2A AR,同时抑制多巴胺代谢酶MAO-B,这种双作用配体可能在帕金森病治疗中提供症状和神经保护作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号