当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lipid-specific interactions determine the organization and dynamics of membrane-active peptide melittin
Soft Matter ( IF 2.9 ) Pub Date : 2020/03/12 , DOI: 10.1039/d0sm00046a Zhixiong Deng 1, 2, 3, 4 , Xuemei Lu 1, 2, 3, 4 , Cheng Xu 1, 2, 3, 4 , Bing Yuan 1, 2, 3, 4, 5 , Kai Yang 1, 2, 3, 4, 5
Soft Matter ( IF 2.9 ) Pub Date : 2020/03/12 , DOI: 10.1039/d0sm00046a Zhixiong Deng 1, 2, 3, 4 , Xuemei Lu 1, 2, 3, 4 , Cheng Xu 1, 2, 3, 4 , Bing Yuan 1, 2, 3, 4, 5 , Kai Yang 1, 2, 3, 4, 5
Affiliation
|
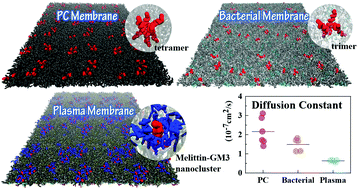
The cell membranes of different cells deviate significantly in lipid compositions and thus provide varying biological environments to modulate the diffusion, organization and the resultant function of biomacromolecules. However, the detailed modulation mechanism remains elusive especially in consideration of the current overuse of the simplified membrane models such as the pure phosphatidylcholine (PC) membrane. In this work, with the typical membrane-active peptide melittin, we demonstrated that a more complicated membrane environment, such as the bacterial (IME) or plasma membrane (PM), would significantly change the organization and dynamics of melittin, by using molecular dynamics simulations as a “computational microscope”. It was found that in these membrane systems, adding melittin would cause a varying degree of reduction in the lateral diffusion of lipids due to the different assembly states of peptides. Melittin tended to aggregate to oligomers in the pure PC membrane, mostly as a tetramer or trimer, while in IME or PM, its degree of oligomerization was significantly reduced. More surprisingly, melittin displayed a strong affinity with ganglioside GM3 in PM, leading to the formation of melittin–GM3 nanoclusters, which hindered its diffusion and further oligomerization. Additionally, small changes in the residue sequence of melittin could modulate the degree or structure of the peptide oligomer. Our work provides a typical example of a study on the organization and dynamics of pore-forming peptides in specific membrane environments and has great significance on the optimization of peptide sequences and the design of helix bundles in the membrane for target biological function.
中文翻译:

脂质特异性相互作用决定膜活性肽蜂毒肽的组织和动力学
不同细胞的细胞膜在脂质组成上有很大的不同,因此提供了不同的生物学环境来调节生物大分子的扩散,组织和最终功能。但是,特别是考虑到当前对简化膜模型(例如纯磷脂酰胆碱(PC)膜)的过度使用,详细的调制机制仍然难以捉摸。在这项工作中,我们使用典型的膜活性肽蜂毒素,证明了更复杂的膜环境,例如细菌(IME)或质膜(PM),将通过分子动力学显着改变蜂毒素的组织和动力学。模拟作为“计算显微镜”。发现在这些膜系统中,由于肽的不同组装状态,添加蜂毒蛋白将导致脂质的横向扩散不同程度的减少。蜂毒肽倾向于在纯PC膜上聚集为低聚物,主要是四聚体或三聚体,而在IME或PM中,其寡聚度显着降低。更令人惊讶的是,蜂毒肽与神经节苷脂GM3在PM中表现出强烈的亲和力,导致蜂毒肽–GM3纳米簇的形成,阻碍了其扩散和进一步的低聚。另外,蜂毒素的残基序列的微小变化可以调节肽寡聚物的程度或结构。
更新日期:2020-04-08
中文翻译:

脂质特异性相互作用决定膜活性肽蜂毒肽的组织和动力学
不同细胞的细胞膜在脂质组成上有很大的不同,因此提供了不同的生物学环境来调节生物大分子的扩散,组织和最终功能。但是,特别是考虑到当前对简化膜模型(例如纯磷脂酰胆碱(PC)膜)的过度使用,详细的调制机制仍然难以捉摸。在这项工作中,我们使用典型的膜活性肽蜂毒素,证明了更复杂的膜环境,例如细菌(IME)或质膜(PM),将通过分子动力学显着改变蜂毒素的组织和动力学。模拟作为“计算显微镜”。发现在这些膜系统中,由于肽的不同组装状态,添加蜂毒蛋白将导致脂质的横向扩散不同程度的减少。蜂毒肽倾向于在纯PC膜上聚集为低聚物,主要是四聚体或三聚体,而在IME或PM中,其寡聚度显着降低。更令人惊讶的是,蜂毒肽与神经节苷脂GM3在PM中表现出强烈的亲和力,导致蜂毒肽–GM3纳米簇的形成,阻碍了其扩散和进一步的低聚。另外,蜂毒素的残基序列的微小变化可以调节肽寡聚物的程度或结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号