当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single-step benzene hydroxylation by cobalt(II) catalysts via a cobalt(III)-hydroperoxo intermediate
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-03-12 , DOI: 10.1039/c9cy02601k Karunanithi Anandababu 1, 2, 3, 4, 5 , Sethuraman Muthuramalingam 1, 2, 3, 4, 5 , Marappan Velusamy 5, 6, 7, 8 , Ramasamy Mayilmurugan 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-03-12 , DOI: 10.1039/c9cy02601k Karunanithi Anandababu 1, 2, 3, 4, 5 , Sethuraman Muthuramalingam 1, 2, 3, 4, 5 , Marappan Velusamy 5, 6, 7, 8 , Ramasamy Mayilmurugan 1, 2, 3, 4, 5
Affiliation
|
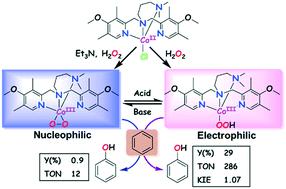
The cobalt(II) complexes of 4N tetradentate ligands have been synthesized and characterized as the catalysts for phenol synthesis in a single step. The molecular structure of the complexes showed a geometry in between square pyramidal and trigonal bipyramidal (τ, 0.49–0.88) with Co–Namine and Co–NPy bond distances of 2.104–2.254 Å and 2.043–2.099 Å, respectively. The complexes exhibited a Co2+/Co3+ redox potential around 0.489–0.500 V vs. Ag/Ag+ in acetonitrile. The complexes catalyzed hydroxylation of benzene using H2O2 (30%) and afforded phenol selectively as the major product. A maximum yield of phenol up to 29% and turnover number (TON) of 286 at 60 °C, and a yield of 19% and TON of 191 at 25 °C are achieved. This is the highest catalytic performance reported using cobalt(II) complexes as catalysts to date. This aromatic hydroxylation presumably proceeded via a cobalt(III)-hydroperoxo species, which was characterized by ESI-MS, and vibrational and electronic spectral methods. The formation of key intermediate [(L)CoIII(OOH)]2+ was accompanied by the appearance of the characteristic O → Co(III) ligand to metal charge transfer (LMCT) transition around 488–686 nm and vibration modes at 832 cm−1 (O–OH) and 564 cm−1 (Co–O). The geometry of one of the catalytically active intermediates was optimized by DFT and its spectral properties were calculated by TD-DFT calculations. These data are comparable to the experimental observations. The kinetic isotope effect (KIE) values (0.98–1.07) support the involvement of cobalt-bound oxygen species as a key intermediate. Isotope-labeling experiments using H218O2 showed an 89% incorporation of 18O, revealing that H2O2 is the main oxygen supplier for phenol formation from benzene. The catalytic efficiencies of cobalt complexes are tuned by ligand architectures via their geometrical configurations and steric properties.
中文翻译:

通过钴(III)-氢过氧中间体通过钴(II)催化剂进行一步式苯羟基化
已经合成了4N四齿配体的钴(II)配合物,并已表征为一步合成苯酚的催化剂。配合物的分子结构显示出方形金字塔形和三角形双锥体之间的几何形状(τ,0.49–0.88),Co–N胺和Co–N Py的键距分别为2.104–2.254Å和2.043–2.099Å。与乙腈中的Ag / Ag +相比,该复合物表现出的Co 2+ / Co 3+氧化还原电位约为0.489–0.500V 。H 2 O 2络合物催化苯的羟基化(30%)并选择性地提供苯酚作为主要产物。在60°C时,苯酚的最大收率可达29%,周转数(TON)为286,在25°C时,收率可达19%,TON值为191。迄今为止,这是使用钴(II)配合物作为催化剂报道的最高催化性能。该芳族羟基化大概是通过钴(III)-氢过氧化物进行的,其特征在于ESI-MS,振动和电子光谱方法。关键中间体[(L)Co III(OOH)] 2+的形成伴随着特征性的O→Co(III)配体到金属电荷转移(LMCT)在488–686 nm处的跃迁以及在832处的振动模式厘米-1(O-OH)和564 cm -1(Co-O)。通过DFT优化了一种催化活性中间体的几何形状,并通过TD-DFT计算来计算其光谱性质。这些数据与实验观察结果相当。动力学同位素效应(KIE)值(0.98–1.07)支持将钴结合的氧作为主要中间体参与。使用H同位素标记实验2 18 ø 2显示了89%的掺入18 O,揭示ħ 2 ö 2是用于从苯酚形成的主要氧气的供应商。钴配合物的催化效率通过配体结构通过 它们的几何构型和空间特性。
更新日期:2020-03-12
中文翻译:

通过钴(III)-氢过氧中间体通过钴(II)催化剂进行一步式苯羟基化
已经合成了4N四齿配体的钴(II)配合物,并已表征为一步合成苯酚的催化剂。配合物的分子结构显示出方形金字塔形和三角形双锥体之间的几何形状(τ,0.49–0.88),Co–N胺和Co–N Py的键距分别为2.104–2.254Å和2.043–2.099Å。与乙腈中的Ag / Ag +相比,该复合物表现出的Co 2+ / Co 3+氧化还原电位约为0.489–0.500V 。H 2 O 2络合物催化苯的羟基化(30%)并选择性地提供苯酚作为主要产物。在60°C时,苯酚的最大收率可达29%,周转数(TON)为286,在25°C时,收率可达19%,TON值为191。迄今为止,这是使用钴(II)配合物作为催化剂报道的最高催化性能。该芳族羟基化大概是通过钴(III)-氢过氧化物进行的,其特征在于ESI-MS,振动和电子光谱方法。关键中间体[(L)Co III(OOH)] 2+的形成伴随着特征性的O→Co(III)配体到金属电荷转移(LMCT)在488–686 nm处的跃迁以及在832处的振动模式厘米-1(O-OH)和564 cm -1(Co-O)。通过DFT优化了一种催化活性中间体的几何形状,并通过TD-DFT计算来计算其光谱性质。这些数据与实验观察结果相当。动力学同位素效应(KIE)值(0.98–1.07)支持将钴结合的氧作为主要中间体参与。使用H同位素标记实验2 18 ø 2显示了89%的掺入18 O,揭示ħ 2 ö 2是用于从苯酚形成的主要氧气的供应商。钴配合物的催化效率通过配体结构通过 它们的几何构型和空间特性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号