npj Digital Medicine ( IF 12.4 ) Pub Date : 2020-03-12 , DOI: 10.1038/s41746-020-0243-5 Katsunori Masaki 1 , Hiroki Tateno 1, 2 , Akihiro Nomura 3, 4, 5 , Tomoyasu Muto 3, 6 , Shin Suzuki 6 , Kohta Satake 3, 6 , Eisuke Hida 7 , Koichi Fukunaga 1
|
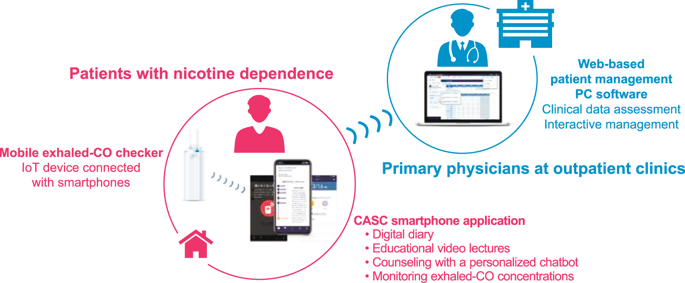
Evidence of the long-term efficacy of digital therapies for smoking cessation that include a smartphone application (app) is limited. In this multi-center randomized controlled trial, we tested the efficacy of a novel digital therapy for smoking cessation: the “CureApp Smoking Cessation (CASC)” system, including a CASC smartphone app, a web-based patient management PC software for primary physicians, and a mobile exhaled carbon monoxide (CO) checker. A total of 584 participants with nicotine dependence were recruited from October 2017 to January 2018, and allocated 1:1 to the CASC intervention group or the control group. Both groups received a standard smoking cessation treatment with pharmacotherapy and counseling for 12 weeks. Meanwhile, the intervention group used the CASC system, and the control group used a control-app without a mobile CO checker, each for 24 weeks. The primary outcome was the biochemically validated continuous abstinence rate (CAR) from weeks 9 to 24. The main secondary outcome was an extended CAR from weeks 9 to 52. Except for 12 participants who did not download or use the apps, 285 participants were assigned to the intervention group, and 287, to the control. CAR from weeks 9 to 24 in the intervention group was significantly higher than that in the control group (63.9% vs. 50.5%; odds ratio [OR], 1.73; 95% confidence interval [CI], 1.24 to 2.42; P = 0.001). The CAR from weeks 9 to 52 was also higher in the intervention group than that in the control group (52.3% vs. 41.5%; OR, 1.55; 95% CI, 1.11 to 2.16; P = 0.010). No specific adverse events caused by the CASC system were reported. Augmenting standard face-to-face counseling and pharmacotherapy with a novel smartphone app, the CASC system significantly improved long-term CARs compared to standard treatment and a minimally supportive control app.
中文翻译:

带有一氧化碳检查器的戒烟智能手机应用程序的随机对照试验
包括智能手机应用程序 (app) 在内的数字戒烟疗法的长期疗效证据有限。在这项多中心随机对照试验中,我们测试了一种新型数字戒烟疗法的功效:“CureApp 戒烟(CASC)”系统,包括 CASC 智能手机应用程序、面向初级医生的基于网络的患者管理 PC 软件,以及移动式呼出一氧化碳 (CO) 检查器。2017年10月至2018年1月共招募584名尼古丁依赖参与者,并按1:1分配至CASC干预组或对照组。两组均接受为期 12 周的标准戒烟治疗,包括药物治疗和咨询。同时,干预组使用 CASC 系统,对照组使用不带移动 CO 检查器的控制应用程序,各持续 24 周。主要结局是第 9 周至第 24 周经生化验证的持续戒断率 (CAR)。主要次要结局是第 9 周至第 52 周的延长 CAR。除了 12 名未下载或使用应用程序的参与者外,还分配了 285 名参与者干预组,287,对照组。干预组第9周至第24周的CAR显着高于对照组(63.9% vs. 50.5%;比值比[OR],1.73;95%置信区间[CI],1.24至2.42;P = 0.001 )。干预组第9周至第52周的CAR也高于对照组(52.3% vs. 41.5%;OR,1.55;95% CI,1.11至2.16;P = 0.010 ) 。没有报告由 CASC 系统引起的具体不良事件。与标准治疗和最低支持控制应用程序相比,CASC 系统通过新型智能手机应用程序增强了标准面对面咨询和药物治疗,显着改善了长期 CAR。











































 京公网安备 11010802027423号
京公网安备 11010802027423号