当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-03-12 , DOI: 10.1021/acs.jnatprod.9b01285 David J Newman 1 , Gordon M Cragg 2
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-03-12 , DOI: 10.1021/acs.jnatprod.9b01285 David J Newman 1 , Gordon M Cragg 2
Affiliation
|
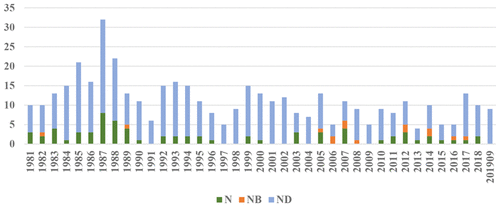
This review is an updated and expanded version of the five prior reviews that were published in this journal in 1997, 2003, 2007, 2012, and 2016. For all approved therapeutic agents, the time frame has been extended to cover the almost 39 years from the first of January 1981 to the 30th of September 2019 for all diseases worldwide and from ∼1946 (earliest so far identified) to the 30th of September 2019 for all approved antitumor drugs worldwide. As in earlier reviews, only the first approval of any drug is counted, irrespective of how many “biosimilars” or added approvals were subsequently identified. As in the 2012 and 2016 reviews, we have continued to utilize our secondary subdivision of a “natural product mimic”, or “NM”, to join the original primary divisions, and the designation “natural product botanical”, or “NB”, to cover those botanical “defined mixtures” now recognized as drug entities by the FDA (and similar organizations). From the data presented in this review, the utilization of natural products and/or synthetic variations using their novel structures, in order to discover and develop the final drug entity, is still alive and well. For example, in the area of cancer, over the time frame from 1946 to 1980, of the 75 small molecules, 40, or 53.3%, are N or ND. In the 1981 to date time frame the equivalent figures for the N* compounds of the 185 small molecules are 62, or 33.5%, though to these can be added the 58 S* and S*/NMs, bringing the figure to 64.9%. In other areas, the influence of natural product structures is quite marked with, as expected from prior information, the anti-infective area being dependent on natural products and their structures, though as can be seen in the review there are still disease areas (shown in Table 2) for which there are no drugs derived from natural products. Although combinatorial chemistry techniques have succeeded as methods of optimizing structures and have been used very successfully in the optimization of many recently approved agents, we are still able to identify only two de novo combinatorial compounds (one of which is a little speculative) approved as drugs in this 39-year time frame, though there is also one drug that was developed using the “fragment-binding methodology” and approved in 2012. We have also added a discussion of candidate drug entities currently in clinical trials as “warheads” and some very interesting preliminary reports on sources of novel antibiotics from Nature due to the absolute requirement for new agents to combat plasmid-borne resistance genes now in the general populace. We continue to draw the attention of readers to the recognition that a significant number of natural product drugs/leads are actually produced by microbes and/or microbial interactions with the “host from whence it was isolated”; thus we consider that this area of natural product research should be expanded significantly.
中文翻译:

从 01/1981 到 09/2019 的近四个十年中作为新药来源的天然产物。
本综述是对 1997 年、2003 年、2007 年、2012 年和 2016 年发表在该杂志上的五篇先前评论的更新和扩展版本。对于所有批准的治疗药物,时间框架已延长至涵盖从1981 年 1 月 1 日至 2019 年 9 月 30 日,适用于全球所有疾病,约 1946 年(迄今为止最早确定的)至 2019 年 9 月 30 日,适用于全球所有批准的抗肿瘤药物。与之前的审查一样,只计算任何药物的首次批准,而不管随后确定了多少“生物仿制药”或新增批准。与 2012 年和 2016 年的审查一样,我们继续利用“天然产品模拟物”或“NM”的二级细分,加入原始的初级细分,以及“天然产品植物”或“NB”的名称,涵盖那些现在被 FDA(和类似组织)认可为药物实体的植物“定义混合物”。从本次审查中提供的数据来看,利用天然产物和/或使用其新结构的合成变异来发现和开发最终药物实体,仍然存在并且很好。例如,在癌症领域,从 1946 年到 1980 年的时间范围内,75 个小分子中有 40 或 53.3% 是 N 或 ND。在 1981 年至今的时间范围内,185 种小分子的 N* 化合物的等效数字为 62,即 33.5%,但可以添加 58 S* 和 S*/NM,使数字达到 64.9%。在其他领域,天然产物结构的影响非常明显,正如先前信息所预期的那样,抗感染领域依赖于天然产物及其结构,尽管从审查中可以看出,仍有一些疾病领域(如表 2 所示)没有来自天然产物的药物。尽管组合化学技术已成功作为优化结构的方法,并在许多最近批准的药物的优化中非常成功地使用,但我们仍然只能识别出两种从头组合化合物(其中一种有点推测性)被批准为药物在这 39 年的时间框架内,尽管还有一种药物是使用“片段结合方法”开发的,并于 2012 年获得批准。我们还添加了对目前作为“弹头”进行临床试验的候选药物实体的讨论,以及一些非常有趣的关于来自 Nature 的新型抗生素来源的初步报告,因为现在普遍需要新的药物来对抗质粒携带的抗性基因民众。我们继续提请读者注意,大量天然药物/铅实际上是由微生物和/或微生物与“宿主从哪里分离出来”的相互作用产生的;因此,我们认为天然产物研究的这一领域应显着扩大。我们继续提请读者注意,大量天然药物/铅实际上是由微生物和/或微生物与“宿主从哪里分离出来”的相互作用产生的;因此,我们认为天然产物研究的这一领域应显着扩大。我们继续提请读者注意,大量天然药物/铅实际上是由微生物和/或微生物与“宿主从哪里分离出来”的相互作用产生的;因此,我们认为天然产物研究的这一领域应显着扩大。
更新日期:2020-04-24
中文翻译:

从 01/1981 到 09/2019 的近四个十年中作为新药来源的天然产物。
本综述是对 1997 年、2003 年、2007 年、2012 年和 2016 年发表在该杂志上的五篇先前评论的更新和扩展版本。对于所有批准的治疗药物,时间框架已延长至涵盖从1981 年 1 月 1 日至 2019 年 9 月 30 日,适用于全球所有疾病,约 1946 年(迄今为止最早确定的)至 2019 年 9 月 30 日,适用于全球所有批准的抗肿瘤药物。与之前的审查一样,只计算任何药物的首次批准,而不管随后确定了多少“生物仿制药”或新增批准。与 2012 年和 2016 年的审查一样,我们继续利用“天然产品模拟物”或“NM”的二级细分,加入原始的初级细分,以及“天然产品植物”或“NB”的名称,涵盖那些现在被 FDA(和类似组织)认可为药物实体的植物“定义混合物”。从本次审查中提供的数据来看,利用天然产物和/或使用其新结构的合成变异来发现和开发最终药物实体,仍然存在并且很好。例如,在癌症领域,从 1946 年到 1980 年的时间范围内,75 个小分子中有 40 或 53.3% 是 N 或 ND。在 1981 年至今的时间范围内,185 种小分子的 N* 化合物的等效数字为 62,即 33.5%,但可以添加 58 S* 和 S*/NM,使数字达到 64.9%。在其他领域,天然产物结构的影响非常明显,正如先前信息所预期的那样,抗感染领域依赖于天然产物及其结构,尽管从审查中可以看出,仍有一些疾病领域(如表 2 所示)没有来自天然产物的药物。尽管组合化学技术已成功作为优化结构的方法,并在许多最近批准的药物的优化中非常成功地使用,但我们仍然只能识别出两种从头组合化合物(其中一种有点推测性)被批准为药物在这 39 年的时间框架内,尽管还有一种药物是使用“片段结合方法”开发的,并于 2012 年获得批准。我们还添加了对目前作为“弹头”进行临床试验的候选药物实体的讨论,以及一些非常有趣的关于来自 Nature 的新型抗生素来源的初步报告,因为现在普遍需要新的药物来对抗质粒携带的抗性基因民众。我们继续提请读者注意,大量天然药物/铅实际上是由微生物和/或微生物与“宿主从哪里分离出来”的相互作用产生的;因此,我们认为天然产物研究的这一领域应显着扩大。我们继续提请读者注意,大量天然药物/铅实际上是由微生物和/或微生物与“宿主从哪里分离出来”的相互作用产生的;因此,我们认为天然产物研究的这一领域应显着扩大。我们继续提请读者注意,大量天然药物/铅实际上是由微生物和/或微生物与“宿主从哪里分离出来”的相互作用产生的;因此,我们认为天然产物研究的这一领域应显着扩大。











































 京公网安备 11010802027423号
京公网安备 11010802027423号