当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Total Synthesis of Inthomycins A, B, and C
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.joc.0c00017 Jae Hyun Kim 1 , Yeonghun Song 1 , Min Jung Kim 1 , Sanghee Kim 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.joc.0c00017 Jae Hyun Kim 1 , Yeonghun Song 1 , Min Jung Kim 1 , Sanghee Kim 1
Affiliation
|
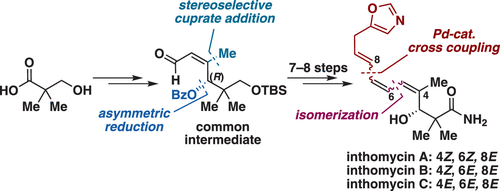
Herein, we report the asymmetric total syntheses of inthomycin antibiotics containing a methylene-interrupted oxazolyl-triene motif. Utilizing the α,β-unsaturated aldehyde as a common intermediate, all three inthomycins A–C were divergently synthesized. The asymmetric ynone reduction provided an R-configured secondary alcohol as in the natural products with high enantioselectivity. The geometrically different triene units for each inthomycin were stereoselectively established via methyl cuprate conjugate addition, isomerization of the α,β-unsaturated aldehyde intermediate, and stereoretentive cross-coupling reactions.
中文翻译:

Inthomycins A,B和C的不对称全合成
在此,我们报告了含有亚甲基中断的恶唑基-三烯基序的合成霉素抗生素的不对称合成。以α,β-不饱和醛为常见中间体,所有三种Inthomycin A–C均以不同方式合成。如天然产物中那样,不对称的炔酮还原提供了R-构型的仲醇,具有高对映选择性。通过添加铜酸甲酯共轭物,α,β-不饱和醛中间体的异构化和立体保持性交叉偶联反应,立体选择性地建立了每一种霉素的几何上不同的三烯单元。
更新日期:2020-03-24
中文翻译:

Inthomycins A,B和C的不对称全合成
在此,我们报告了含有亚甲基中断的恶唑基-三烯基序的合成霉素抗生素的不对称合成。以α,β-不饱和醛为常见中间体,所有三种Inthomycin A–C均以不同方式合成。如天然产物中那样,不对称的炔酮还原提供了R-构型的仲醇,具有高对映选择性。通过添加铜酸甲酯共轭物,α,β-不饱和醛中间体的异构化和立体保持性交叉偶联反应,立体选择性地建立了每一种霉素的几何上不同的三烯单元。











































 京公网安备 11010802027423号
京公网安备 11010802027423号