当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst-Free Double CH-Functionalization of Quinolines with Phosphine Oxides via Two SNHAr Reaction Sequences
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-03-25 , DOI: 10.1021/acs.joc.0c00084 Boris A. Trofimov 1 , Pavel A. Volkov 1 , Anton A. Telezhkin 1 , Kseniya O. Khrapova 1 , Nina I. Ivanova 1 , Alexander I. Albanov 1 , Nina K. Gusarova 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-03-25 , DOI: 10.1021/acs.joc.0c00084 Boris A. Trofimov 1 , Pavel A. Volkov 1 , Anton A. Telezhkin 1 , Kseniya O. Khrapova 1 , Nina I. Ivanova 1 , Alexander I. Albanov 1 , Nina K. Gusarova 1
Affiliation
|
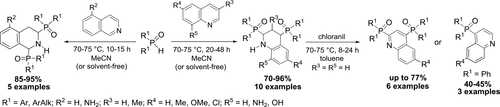
Quinolines undergo catalyst-free double CH-functionalization upon treatment with secondary phosphine oxides (70–75 °C, 20–48 h) followed by oxidation of the intermediate 2,4-bisphosphoryltetrahydroquinolines with chloranil. The yields of the target 2,4-bisphosphorylated quinolines are up to 77%. Thus, a double-SNHAr reaction sequence in the same molecule of quinoline has been realized. In the case of 2,4-bisphenylphosphoryltetrahydroquinolines, the aromatization occurs with elimination of one molecule of diphenylphosphine oxide to afford the products of monofunctionalization, 4-diphenylphosphorylquinolines, in 40–45% yields.
中文翻译:

通过两个S N H Ar反应序列用氧化膦将喹啉进行无催化剂的双CH功能化
喹啉经过仲膦氧化物(70-75°C,20-48 h)处理后,经过无催化剂的双CH官能化,然后用氯苯腈氧化中间体2,4-双磷酰基四氢喹啉。目标2,4-双磷酸化喹啉的产率高达77%。因此,已经实现了在相同的喹啉分子中的双S N H Ar反应序列。在2,4-双苯基磷酰基四氢喹啉的情况下,通过消除一分子的二苯基氧化膦来进行芳构化,从而以40-45%的产率提供单官能化的产物4-二苯基磷酰基喹啉。
更新日期:2020-03-26
中文翻译:

通过两个S N H Ar反应序列用氧化膦将喹啉进行无催化剂的双CH功能化
喹啉经过仲膦氧化物(70-75°C,20-48 h)处理后,经过无催化剂的双CH官能化,然后用氯苯腈氧化中间体2,4-双磷酰基四氢喹啉。目标2,4-双磷酸化喹啉的产率高达77%。因此,已经实现了在相同的喹啉分子中的双S N H Ar反应序列。在2,4-双苯基磷酰基四氢喹啉的情况下,通过消除一分子的二苯基氧化膦来进行芳构化,从而以40-45%的产率提供单官能化的产物4-二苯基磷酰基喹啉。











































 京公网安备 11010802027423号
京公网安备 11010802027423号