当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Meayamycin B
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-03-23 , DOI: 10.1021/acs.joc.9b03370 Robert K Bressin 1 , Sami Osman 1 , Ivanna Pohorilets 1 , Upamanyu Basu 1 , Kazunori Koide 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-03-23 , DOI: 10.1021/acs.joc.9b03370 Robert K Bressin 1 , Sami Osman 1 , Ivanna Pohorilets 1 , Upamanyu Basu 1 , Kazunori Koide 1
Affiliation
|
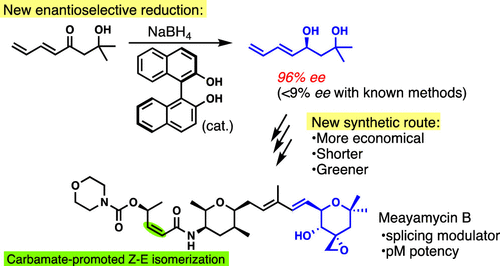
Meayamycin B is currently the most potent modulator of the splicing factor 3b subunit 1 and used by dozens of research groups. However, current supply for this natural product analogue is limited because of the lengthy synthetic scheme. Here, we report a more concise, more cost-effective, and greener synthesis of this compound by developing and employing a novel asymmetric reduction of a prochiral enone to afford an allylic alcohol with high enantioselectivity. In addition to this reaction, this synthesis highlights a scalable Mukaiyama aldol reaction, Nicolaou-type epoxide opening reaction, stereoselective Corey–Chaykovsky-type reaction, and a modified Horner–Wadsworth–Emmons Z-selective olefination. We also discuss a Z–E isomerization during the α,β-unsaturated amide formation. The new synthesis of meayamycin B consists of 11 steps in the longest linear sequence and 24 total steps.
中文翻译:

美亚霉素B的全合成
Meayamycin B是目前最有效的剪接因子3b亚基1的调节剂,已有数十个研究小组使用。然而,由于冗长的合成方案,该天然产物类似物的电流供应受到限制。在这里,我们报道了通过开发和采用前手性烯酮的新型不对称还原以提供具有高对映选择性的烯丙基醇,该化合物更简洁,更具成本效益和更绿色的合成。除此反应外,该合成还突出显示了可扩展的Mukaiyama羟醛反应,Nicolaou型环氧化物开环反应,立体选择性Corey–Chaykovsky型反应和改良的Horner–Wadsworth–Emmons Z选择烯烃。我们还将讨论Z – Eα,β-不饱和酰胺形成过程中的异构化。meayamycin B的新合成包括最长线性序列中的11个步骤和总共24个步骤。
更新日期:2020-03-24
中文翻译:

美亚霉素B的全合成
Meayamycin B是目前最有效的剪接因子3b亚基1的调节剂,已有数十个研究小组使用。然而,由于冗长的合成方案,该天然产物类似物的电流供应受到限制。在这里,我们报道了通过开发和采用前手性烯酮的新型不对称还原以提供具有高对映选择性的烯丙基醇,该化合物更简洁,更具成本效益和更绿色的合成。除此反应外,该合成还突出显示了可扩展的Mukaiyama羟醛反应,Nicolaou型环氧化物开环反应,立体选择性Corey–Chaykovsky型反应和改良的Horner–Wadsworth–Emmons Z选择烯烃。我们还将讨论Z – Eα,β-不饱和酰胺形成过程中的异构化。meayamycin B的新合成包括最长线性序列中的11个步骤和总共24个步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号