当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and docking study of benzimidazole–triazolothiadiazine hybrids as aromatase inhibitors
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2020-03-11 , DOI: 10.1002/ardp.202000008 Ulviye Acar Çevik 1, 2 , Begüm N Sağlık 1, 2 , Derya Osmaniye 1, 2 , Serkan Levent 1, 2 , Betül Kaya Çavuşoğlu 1, 2 , Abdullah B Karaduman 3 , Yusuf Özkay 1, 2 , Zafer A Kaplancıklı 1
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2020-03-11 , DOI: 10.1002/ardp.202000008 Ulviye Acar Çevik 1, 2 , Begüm N Sağlık 1, 2 , Derya Osmaniye 1, 2 , Serkan Levent 1, 2 , Betül Kaya Çavuşoğlu 1, 2 , Abdullah B Karaduman 3 , Yusuf Özkay 1, 2 , Zafer A Kaplancıklı 1
Affiliation
|
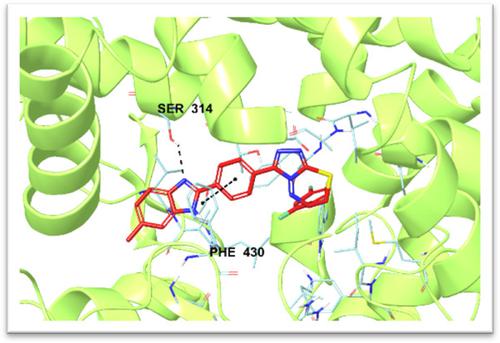
Aromatase is involved in the biosynthesis of estrogen and thus is a critical target for breast cancer. In this study, to identify new aromatase enzyme inhibitors, seven 3‐[4‐(5‐methyl‐1H‐benzo[d]imidazol‐2‐yl)phenyl]‐6‐(substituted phenyl)‐7H‐[1,2,4]triazolo[3,4‐b][1,3,4]thiadiazine derivatives were synthesized. First, a 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay was performed to determine the inhibitory activity of the synthesized compounds on the MCF‐7 cell line. The aromatase inhibitory activity was determined for the active compounds 5b, 5c, 5e, and 5g on the MCF‐7 cell line. Compound 5g showed significant aromatase inhibitory activity (IC50 = 0.037 ± 0.001 µM). Interestingly, this compound, which bears a difluoro substituent at positions 2 and 4 of the phenyl ring, displayed the most potent aromatase inhibitory activity without significant cytotoxicity to a normal healthy cell line (NIH3T3). Furthermore, the interactions between the best active compounds and the active site of the enzyme were analyzed through a docking study. The results of this study determined that benzimidazole–triazolothiadiazine derivatives are promising compounds that should be further developed as a novel class of aromatase inhibitors.
中文翻译:

苯并咪唑-三唑噻二嗪杂化物作为芳香酶抑制剂的合成与对接研究
芳香酶参与雌激素的生物合成,因此是乳腺癌的关键靶点。在本研究中,为了鉴定新的芳香酶抑制剂,7 个 3-[4-(5-methyl-1H-benzo[d]imidazol-2-yl)phenyl]-6-(已取代的苯基)-7H-[1,2 ,4]三唑并[3,4-b][1,3,4]噻二嗪衍生物的合成。首先,进行了 3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四唑 (MTT) 测定以确定合成化合物对 MCF-7 细胞系的抑制活性。测定了活性化合物 5b、5c、5e 和 5g 对 MCF-7 细胞系的芳香酶抑制活性。化合物 5g 显示出显着的芳香酶抑制活性 (IC50 = 0.037 ± 0.001 µM)。有趣的是,这种在苯环的 2 和 4 位带有二氟取代基的化合物,显示出最有效的芳香酶抑制活性,对正常健康细胞系 (NIH3T3) 没有显着的细胞毒性。此外,通过对接研究分析了最佳活性化合物与酶活性位点之间的相互作用。这项研究的结果确定苯并咪唑-三唑噻二嗪衍生物是有前途的化合物,应进一步开发为一类新型的芳香酶抑制剂。
更新日期:2020-03-11
中文翻译:

苯并咪唑-三唑噻二嗪杂化物作为芳香酶抑制剂的合成与对接研究
芳香酶参与雌激素的生物合成,因此是乳腺癌的关键靶点。在本研究中,为了鉴定新的芳香酶抑制剂,7 个 3-[4-(5-methyl-1H-benzo[d]imidazol-2-yl)phenyl]-6-(已取代的苯基)-7H-[1,2 ,4]三唑并[3,4-b][1,3,4]噻二嗪衍生物的合成。首先,进行了 3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四唑 (MTT) 测定以确定合成化合物对 MCF-7 细胞系的抑制活性。测定了活性化合物 5b、5c、5e 和 5g 对 MCF-7 细胞系的芳香酶抑制活性。化合物 5g 显示出显着的芳香酶抑制活性 (IC50 = 0.037 ± 0.001 µM)。有趣的是,这种在苯环的 2 和 4 位带有二氟取代基的化合物,显示出最有效的芳香酶抑制活性,对正常健康细胞系 (NIH3T3) 没有显着的细胞毒性。此外,通过对接研究分析了最佳活性化合物与酶活性位点之间的相互作用。这项研究的结果确定苯并咪唑-三唑噻二嗪衍生物是有前途的化合物,应进一步开发为一类新型的芳香酶抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号