当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Biological Screening of New Lawson Derivatives as Selective Substrate-Based Inhibitors of Cytochrome bo3 Ubiquinol Oxidase from Escherichia coli.
ChemMedChem ( IF 3.4 ) Pub Date : 2020-03-11 , DOI: 10.1002/cmdc.201900707 Isam Elamri 1 , Melanie Radloff 2 , Katharina F Hohmann 1 , Vijaykumar D Nimbarte 1 , Hamid R Nasiri 1 , Michael Bolte 3 , Schara Safarian 2 , Hartmut Michel 2 , Harald Schwalbe 1
ChemMedChem ( IF 3.4 ) Pub Date : 2020-03-11 , DOI: 10.1002/cmdc.201900707 Isam Elamri 1 , Melanie Radloff 2 , Katharina F Hohmann 1 , Vijaykumar D Nimbarte 1 , Hamid R Nasiri 1 , Michael Bolte 3 , Schara Safarian 2 , Hartmut Michel 2 , Harald Schwalbe 1
Affiliation
|
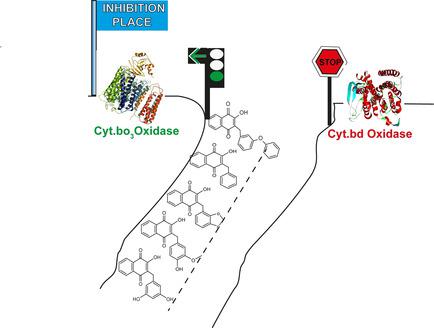
The respiratory chain of Escherichia coli contains two different types of terminal oxidase that are differentially regulated as a response to changing environmental conditions. These oxidoreductases catalyze the reduction of molecular oxygen to water and contribute to the proton motive force. The cytochrome bo 3 oxidase (cyt bo 3) acts as the primary terminal oxidase under atmospheric oxygen levels, whereas the bd‐type oxidase is most abundant under microaerobic conditions. In E. coli , both types of respiratory terminal oxidase (HCO and bd‐type) use ubiquinol‐8 as electron donor. Here, we assess the inhibitory potential of newly designed and synthesized 3‐alkylated Lawson derivatives through L‐proline‐catalyzed three‐component reductive alkylation (TCRA). The inhibitory effects of these Lawson derivatives on the terminal oxidases of E. coli (cyt bo 3 and cyt bd‐I) were tested potentiometrically. Four compounds were able to reduce the oxidoreductase activity of cyt bo 3 by more than 50 % without affecting the cyt bd‐ I activity. Moreover, two inhibitors for both cyt bo 3 and cyt bd‐I oxidase could be identified. Based on molecular‐docking simulations, we propose binding modes of the new Lawson inhibitors. The molecular fragment benzyl enhances the inhibitory potential and selectivity for cyt bo 3, whereas heterocycles reduce this effect. This work extends the library of 3‐alkylated Lawson derivatives as selective inhibitors for respiratory oxidases and provides molecular probes for detailed investigations of the mechanisms of respiratory‐chain enzymes of E. coli .
中文翻译:

新型劳森衍生物的合成和生物学筛选,作为大肠杆菌中细胞色素bo3泛醇氧化酶的选择性底物抑制剂。
大肠杆菌的呼吸链包含两种不同类型的末端氧化酶,它们可根据环境条件的变化而受到不同的调节。这些氧化还原酶催化分子氧还原为水并有助于质子原动力。在大气氧水平下,细胞色素bo 3氧化酶(cyt bo 3)充当主要的末端氧化酶,而bd型氧化酶在微需氧条件下含量最高。在大肠杆菌中,两种类型的呼吸末氧化酶(HCO和bd式)使用泛醇8作为电子供体。在这里,我们通过L-脯氨酸催化的三组分还原烷基化(TCRA)评估了新设计和合成的3-烷基化Lawson衍生物的抑制潜力。电位滴定法测试了这些Lawson衍生物对大肠杆菌末端氧化酶(cyt bo 3和cyt bd -1)的抑制作用。四种化合物能够将cyt bo 3的氧化还原酶活性降低50%以上,而不会影响cyt bd- I活性。此外,cyt bo 3和cyt bd的两种抑制剂‐I氧化酶可以被识别。基于分子对接模拟,我们提出了新的Lawson抑制剂的结合模式。分子片段苄基增强了对cyt bo 3的抑制能力和选择性,而杂环降低了这种作用。这项工作扩展了3烷基化Lawson衍生物作为呼吸道氧化酶的选择性抑制剂的库,并为详细研究大肠杆菌呼吸链酶的机制提供了分子探针。
更新日期:2020-03-11
中文翻译:

新型劳森衍生物的合成和生物学筛选,作为大肠杆菌中细胞色素bo3泛醇氧化酶的选择性底物抑制剂。
大肠杆菌的呼吸链包含两种不同类型的末端氧化酶,它们可根据环境条件的变化而受到不同的调节。这些氧化还原酶催化分子氧还原为水并有助于质子原动力。在大气氧水平下,细胞色素bo 3氧化酶(cyt bo 3)充当主要的末端氧化酶,而bd型氧化酶在微需氧条件下含量最高。在大肠杆菌中,两种类型的呼吸末氧化酶(HCO和bd式)使用泛醇8作为电子供体。在这里,我们通过L-脯氨酸催化的三组分还原烷基化(TCRA)评估了新设计和合成的3-烷基化Lawson衍生物的抑制潜力。电位滴定法测试了这些Lawson衍生物对大肠杆菌末端氧化酶(cyt bo 3和cyt bd -1)的抑制作用。四种化合物能够将cyt bo 3的氧化还原酶活性降低50%以上,而不会影响cyt bd- I活性。此外,cyt bo 3和cyt bd的两种抑制剂‐I氧化酶可以被识别。基于分子对接模拟,我们提出了新的Lawson抑制剂的结合模式。分子片段苄基增强了对cyt bo 3的抑制能力和选择性,而杂环降低了这种作用。这项工作扩展了3烷基化Lawson衍生物作为呼吸道氧化酶的选择性抑制剂的库,并为详细研究大肠杆菌呼吸链酶的机制提供了分子探针。



























 京公网安备 11010802027423号
京公网安备 11010802027423号