Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Toxoplasma infection induces microglia-neuron contact and the loss of perisomatic inhibitory synapses.
Glia ( IF 5.4 ) Pub Date : 2020-03-11 , DOI: 10.1002/glia.23816 Gabriela L Carrillo 1, 2 , Valerie A Ballard 1, 3 , Taylor Glausen 4 , Zack Boone 1, 5 , Joseph Teamer 1, 6 , Cyrus L Hinkson 1, 7 , Elizabeth A Wohlfert 4 , Ira J Blader 4 , Michael A Fox 1, 5, 8, 9
Glia ( IF 5.4 ) Pub Date : 2020-03-11 , DOI: 10.1002/glia.23816 Gabriela L Carrillo 1, 2 , Valerie A Ballard 1, 3 , Taylor Glausen 4 , Zack Boone 1, 5 , Joseph Teamer 1, 6 , Cyrus L Hinkson 1, 7 , Elizabeth A Wohlfert 4 , Ira J Blader 4 , Michael A Fox 1, 5, 8, 9
Affiliation
|
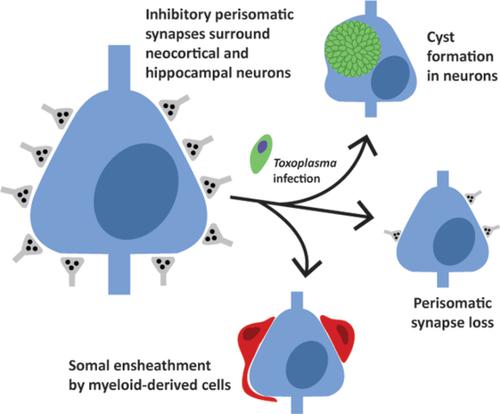
Infection and inflammation within the brain induces changes in neuronal connectivity and function. The intracellular protozoan parasite, Toxoplasma gondii , is one pathogen that infects the brain and can cause encephalitis and seizures. Persistent infection by this parasite is also associated with behavioral alterations and an increased risk for developing psychiatric illness, including schizophrenia. Current evidence from studies in humans and mouse models suggest that both seizures and schizophrenia result from a loss or dysfunction of inhibitory synapses. In line with this, we recently reported that persistent T. gondii infection alters the distribution of glutamic acid decarboxylase 67 (GAD67), an enzyme that catalyzes GABA synthesis in inhibitory synapses. These changes could reflect a redistribution of presynaptic machinery in inhibitory neurons or a loss of inhibitory nerve terminals. To directly assess the latter possibility, we employed serial block face scanning electron microscopy (SBFSEM) and quantified inhibitory perisomatic synapses in neocortex and hippocampus following parasitic infection. Not only did persistent infection lead to a significant loss of perisomatic synapses, it induced the ensheathment of neuronal somata by myeloid‐derived cells. Immunohistochemical, genetic, and ultrastructural analyses revealed that these myeloid‐derived cells included activated microglia. Finally, ultrastructural analysis identified myeloid‐derived cells enveloping perisomatic nerve terminals, suggesting they may actively displace or phagocytose synaptic elements. Thus, these results suggest that activated microglia contribute to perisomatic inhibitory synapse loss following parasitic infection and offer a novel mechanism as to how persistent T. gondii infection may contribute to both seizures and psychiatric illness.
中文翻译:

弓形虫感染诱导小胶质细胞-神经元接触和体周抑制性突触的丧失。
大脑内的感染和炎症会引起神经元连接和功能的变化。细胞内原生动物寄生虫弓形虫是一种感染大脑的病原体,可引起脑炎和癫痫发作。这种寄生虫的持续感染还与行为改变和患精神疾病(包括精神分裂症)的风险增加有关。目前对人类和小鼠模型的研究证据表明,癫痫发作和精神分裂症都是由抑制性突触的丧失或功能障碍引起的。与此相一致,我们最近报道,持续的弓形虫感染改变了谷氨酸脱羧酶 67 (GAD67) 的分布,谷氨酸脱羧酶 67 是一种催化抑制性突触中 GABA 合成的酶。这些变化可能反映了抑制性神经元中突触前机制的重新分配或抑制性神经末梢的丧失。为了直接评估后一种可能性,我们采用了串行块面扫描电子显微镜(SBFSEM)并量化了寄生虫感染后新皮质和海马体中的抑制性周突触。持续感染不仅导致体周突触的显着损失,而且还诱导髓源性细胞包裹神经元体。免疫组织化学、遗传和超微结构分析表明,这些骨髓来源的细胞包括活化的小胶质细胞。最后,超微结构分析发现髓源性细胞包裹体周神经末梢,表明它们可能主动取代或吞噬突触元件。因此,这些结果表明,激活的小胶质细胞会导致寄生虫感染后体周抑制性突触丧失,并为持续性弓形虫感染如何导致癫痫发作和精神疾病提供了一种新机制。
更新日期:2020-03-11
中文翻译:

弓形虫感染诱导小胶质细胞-神经元接触和体周抑制性突触的丧失。
大脑内的感染和炎症会引起神经元连接和功能的变化。细胞内原生动物寄生虫弓形虫是一种感染大脑的病原体,可引起脑炎和癫痫发作。这种寄生虫的持续感染还与行为改变和患精神疾病(包括精神分裂症)的风险增加有关。目前对人类和小鼠模型的研究证据表明,癫痫发作和精神分裂症都是由抑制性突触的丧失或功能障碍引起的。与此相一致,我们最近报道,持续的弓形虫感染改变了谷氨酸脱羧酶 67 (GAD67) 的分布,谷氨酸脱羧酶 67 是一种催化抑制性突触中 GABA 合成的酶。这些变化可能反映了抑制性神经元中突触前机制的重新分配或抑制性神经末梢的丧失。为了直接评估后一种可能性,我们采用了串行块面扫描电子显微镜(SBFSEM)并量化了寄生虫感染后新皮质和海马体中的抑制性周突触。持续感染不仅导致体周突触的显着损失,而且还诱导髓源性细胞包裹神经元体。免疫组织化学、遗传和超微结构分析表明,这些骨髓来源的细胞包括活化的小胶质细胞。最后,超微结构分析发现髓源性细胞包裹体周神经末梢,表明它们可能主动取代或吞噬突触元件。因此,这些结果表明,激活的小胶质细胞会导致寄生虫感染后体周抑制性突触丧失,并为持续性弓形虫感染如何导致癫痫发作和精神疾病提供了一种新机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号