Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Flavodoxin hydroquinone provides electrons for the ATP‐dependent reactivation of protein‐bound corrinoid cofactors
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-03-11 , DOI: 10.1111/febs.15290 Lena Kißling 1 , Yvonne Greiser 1 , Hendrike Dürichen 1 , Sandra Studenik 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-03-11 , DOI: 10.1111/febs.15290 Lena Kißling 1 , Yvonne Greiser 1 , Hendrike Dürichen 1 , Sandra Studenik 1
Affiliation
|
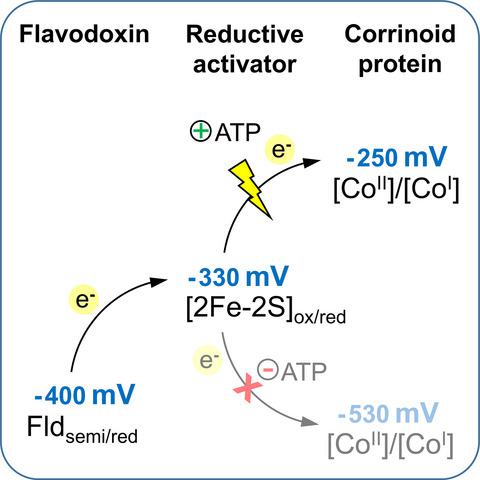
Corrinoid‐dependent enzyme systems rely on the super‐reduced state of the protein‐bound corrinoid cofactor to be functional, for example, in methyl transfer reactions. Due to the low redox potential of the [CoII]/[CoI] couple, autoxidation of the corrinoid cofactor occurs and leads to the formation of the inactive [CoII]‐state. For the reactivation, which is an energy‐demanding process, electrons have to be transferred from a physiological donor to the corrinoid cofactor by the help of a reductive activator protein. In this study, we identified reduced flavodoxin as electron donor for the ATP‐dependent reduction of protein‐bound corrinoid cofactors of bacterial O‐demethylase enzyme systems. Reduced flavodoxin was generated enzymatically using pyruvate:ferredoxin/flavodoxin oxidoreductase rather than hydrogenase. Two of the four flavodoxins identified in Acetobacterium dehalogenans and Desulfitobacterium hafniense DCB‐2 were functional in supplying electrons for corrinoid reduction. They exhibited a midpoint potential of about −400 mV (ESHE, pH 7.5) for the semiquinone/hydroquinone transition. Reduced flavodoxin could be replaced by reduced clostridial ferredoxin. It was shown that the low‐potential electrons of reduced flavodoxin are first transferred to the iron‐sulfur cluster of the reductive activator and finally to the protein‐bound corrinoid cofactor. This study further highlights the importance of reduced flavodoxin, which allows maintaining a variety of enzymatic reaction cycles by delivering low‐potential electrons.
中文翻译:

Flavodoxin对苯二酚为结合蛋白的类rinrin辅因子的ATP依赖性再活化提供电子
Corrinoid依赖的酶系统依赖于蛋白质结合的corrinoid辅因子的超还原状态,例如在甲基转移反应中起作用。由于[Co II ] / [Co I ]对的氧化还原电势低,因此发生了类固醇辅因子的自氧化反应,并导致形成了无活性的[Co II ]-态。要进行重新激活,这是一个耗能的过程,必须通过还原性激活蛋白将电子从生理供体转移到类Corrinoid辅因子上。在这项研究中,我们确定还原型黄酮毒素是电子供体,可帮助细菌O依赖于ATP的结合蛋白类视黄体辅助因子减少脱甲基酶系统。使用丙酮酸:铁氧还蛋白/黄酮毒素氧化还原酶而不是氢酶以酶促方式产生黄酮毒素的还原。在卤化醋杆菌和哈弗脱硫杆菌DCB-2中鉴定出的四种黄酮毒素中的两种在提供电子以减少类胡萝卜素方面起作用。它们表现出约-400 mV的中点电位(E SHE,pH 7.5)进行半醌/氢醌过渡。减少的黄酮毒素可用减少的梭菌铁氧还蛋白代替。结果表明,还原型黄酮毒素的低电位电子首先被转移到还原性活化剂的铁硫簇中,最后被转移到与蛋白质结合的类corrinoid辅因子上。这项研究进一步强调了还原黄酮毒素的重要性,黄酮毒素可以通过传递低电势的电子来维持各种酶促反应周期。
更新日期:2020-03-11
中文翻译:

Flavodoxin对苯二酚为结合蛋白的类rinrin辅因子的ATP依赖性再活化提供电子
Corrinoid依赖的酶系统依赖于蛋白质结合的corrinoid辅因子的超还原状态,例如在甲基转移反应中起作用。由于[Co II ] / [Co I ]对的氧化还原电势低,因此发生了类固醇辅因子的自氧化反应,并导致形成了无活性的[Co II ]-态。要进行重新激活,这是一个耗能的过程,必须通过还原性激活蛋白将电子从生理供体转移到类Corrinoid辅因子上。在这项研究中,我们确定还原型黄酮毒素是电子供体,可帮助细菌O依赖于ATP的结合蛋白类视黄体辅助因子减少脱甲基酶系统。使用丙酮酸:铁氧还蛋白/黄酮毒素氧化还原酶而不是氢酶以酶促方式产生黄酮毒素的还原。在卤化醋杆菌和哈弗脱硫杆菌DCB-2中鉴定出的四种黄酮毒素中的两种在提供电子以减少类胡萝卜素方面起作用。它们表现出约-400 mV的中点电位(E SHE,pH 7.5)进行半醌/氢醌过渡。减少的黄酮毒素可用减少的梭菌铁氧还蛋白代替。结果表明,还原型黄酮毒素的低电位电子首先被转移到还原性活化剂的铁硫簇中,最后被转移到与蛋白质结合的类corrinoid辅因子上。这项研究进一步强调了还原黄酮毒素的重要性,黄酮毒素可以通过传递低电势的电子来维持各种酶促反应周期。











































 京公网安备 11010802027423号
京公网安备 11010802027423号