Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zinc modulation of proton currents in a new voltage‐gated proton channel suggests a mechanism of inhibition
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-03-11 , DOI: 10.1111/febs.15291 Gustavo Chaves 1 , Stefanie Bungert‐Plümke 2 , Arne Franzen 2 , Iryna Mahorivska 1 , Boris Musset 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-03-11 , DOI: 10.1111/febs.15291 Gustavo Chaves 1 , Stefanie Bungert‐Plümke 2 , Arne Franzen 2 , Iryna Mahorivska 1 , Boris Musset 1
Affiliation
|
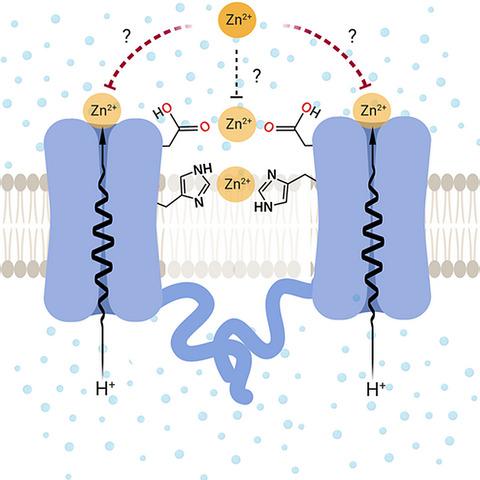
The HV1 voltage‐gated proton (HV1) channel is a key component of the cellular proton extrusion machinery and is pivotal for charge compensation during the respiratory burst of phagocytes. The best‐described physiological inhibitor of HV1 is Zn2+. Externally applied ZnCl2 drastically reduces proton currents reportedly recorded in Homo sapiens, Rattus norvegicus, Mus musculus, Oryctolagus cuniculus, Rana esculenta, Helix aspersa, Ciona intestinalis, Coccolithus pelagicus, Emiliania huxleyi, Danio rerio, Helisoma trivolvis, and Lingulodinium polyedrum, but with considerable species variability. Here, we report the effects of Zn2+ and Cd2+ on HV1 from Nicoletia phytophila, NpHV1. We introduced mutations at potential Zn2+ coordination sites and measured Zn2+ inhibition in different extracellular pH, with Zn2+ concentrations up to 1000 μm. Zn2+ inhibition in NpHV1 was quantified by the slowing of the activation time constant and a positive shift of the conductance–voltage curve. Replacing aspartate in the S3‐S4 loop with histidine (D145H) enhanced both the slowing of activation kinetics and the shift in the voltage–conductance curve, such that Zn2+ inhibition closely resembled that of the human channel. Histidine is much more effective than aspartate in coordinating Zn2+ in the S3‐S4 linker. A simple Hodgkin Huxley model of NpHV1 suggests a decrease in the opening rate if it is inhibited by zinc or cadmium. Limiting slope measurements and high‐resolution clear native gel electrophoresis (hrCNE) confirmed that NpHV1 functions as a dimer. The data support the hypothesis that zinc is coordinated in between the dimer instead of the monomer. Zinc coordination sites may be potential targets for drug development.
中文翻译:

新的电压门控质子通道中质子电流的锌调制表明了一种抑制机制
H V 1电压门控质子(H V 1)通道是细胞质子挤出机制的关键组成部分,对于吞噬细胞呼吸爆发期间的电荷补偿至关重要。H V 1的最佳生理抑制剂是Zn 2+。外部施加的ZnCl 2显着地降低了据说记录在质子电流智人,褐家鼠,小家鼠,家兔,蛙星虫,花园蜗牛,玻璃海鞘,Coccolithus pelagicus,-贺,斑马鱼,Helisoma trivolvis,并Lingulodinium polyedrum,但具有相当大的变异品种。这里,我们报告的Zn的效果2+和Cd 2+上ħ V 1从Nicoletia phytophila,NPH V 1。我们在潜在的Zn引入的突变2+的配位点和测量的Zn 2+在不同细胞外pH抑制作用,有Zn 2 +浓度高达1000μ米。NpH V中的Zn 2+抑制通过激活时间常数的减慢和电导-电压曲线的正移来量化1。用组氨酸(D145H)代替S3-S4环中的天冬氨酸,既可以激活速度的减慢,又可以改变电压-电导率曲线,从而使Zn 2+的抑制作用与人类通道的抑制作用非常相似。组氨酸比S3-S4接头中的Zn 2+配比天冬氨酸更有效。一个简单的NpH V 1的霍奇金·赫x黎(Hodgkin Huxley)模型表明,如果锌或镉抑制了开孔率,则会降低开孔率。极限斜率测量和高分辨率清晰的天然凝胶电泳(hrCNE)证实NpH V1用作二聚体。数据支持锌在二聚体而不是单体之间配位的假说。锌协调位点可能是药物开发的潜在目标。
更新日期:2020-03-11
中文翻译:

新的电压门控质子通道中质子电流的锌调制表明了一种抑制机制
H V 1电压门控质子(H V 1)通道是细胞质子挤出机制的关键组成部分,对于吞噬细胞呼吸爆发期间的电荷补偿至关重要。H V 1的最佳生理抑制剂是Zn 2+。外部施加的ZnCl 2显着地降低了据说记录在质子电流智人,褐家鼠,小家鼠,家兔,蛙星虫,花园蜗牛,玻璃海鞘,Coccolithus pelagicus,-贺,斑马鱼,Helisoma trivolvis,并Lingulodinium polyedrum,但具有相当大的变异品种。这里,我们报告的Zn的效果2+和Cd 2+上ħ V 1从Nicoletia phytophila,NPH V 1。我们在潜在的Zn引入的突变2+的配位点和测量的Zn 2+在不同细胞外pH抑制作用,有Zn 2 +浓度高达1000μ米。NpH V中的Zn 2+抑制通过激活时间常数的减慢和电导-电压曲线的正移来量化1。用组氨酸(D145H)代替S3-S4环中的天冬氨酸,既可以激活速度的减慢,又可以改变电压-电导率曲线,从而使Zn 2+的抑制作用与人类通道的抑制作用非常相似。组氨酸比S3-S4接头中的Zn 2+配比天冬氨酸更有效。一个简单的NpH V 1的霍奇金·赫x黎(Hodgkin Huxley)模型表明,如果锌或镉抑制了开孔率,则会降低开孔率。极限斜率测量和高分辨率清晰的天然凝胶电泳(hrCNE)证实NpH V1用作二聚体。数据支持锌在二聚体而不是单体之间配位的假说。锌协调位点可能是药物开发的潜在目标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号