当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Suzuki‐Miyaura Cross‐Coupling of Amides using Well‐Defined, Air‐Stable [(PR3)2Pd(II)X2] Precatalysts
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-04-03 , DOI: 10.1002/adsc.202000122 Siyue Ma 1 , Tongliang Zhou 1 , Guangchen Li 1 , Michal Szostak 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-04-03 , DOI: 10.1002/adsc.202000122 Siyue Ma 1 , Tongliang Zhou 1 , Guangchen Li 1 , Michal Szostak 1
Affiliation
|
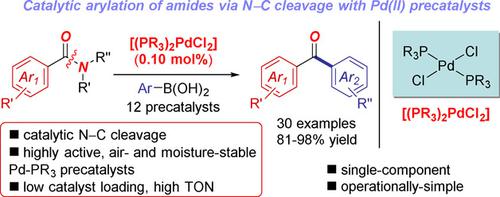
A versatile method for the Suzuki‐Miyaura cross‐coupling of amides using highly active, well‐defined, and air‐stable Pd−phosphine precatalysts is reported. Most notably, the method represents the first example of using practical and operationally‐simple Pd(II)−phosphine precatalysts in the emerging amide bond cross‐coupling manifold. The reactions are efficient at 0.10 mol% loading, furnishing biaryl ketones with high chemoselectivity for N−C(O) bond cleavage. This versatile method enables for the first time to achieve Pd−phosphine‐catalyzed cross‐coupling of amides at ppm loading. This C−N cross‐coupling can be used to efficiently furnish pharmaceutical intermediates by orthogonal Pd‐catalyzed cross‐couplings. We fully expect that operationally‐simple [(PR3)2Pd(II)X2] precatalysts as effective triggers for N−C(O) cross‐coupling will be of broad synthetic and catalytic interest.
中文翻译:

Suzuki-Miyaura使用良好定义的,稳定的[[PR3)2Pd(II)X2]预催化剂对酰胺进行交叉偶联
报道了一种使用高活性,定义明确且空气稳定的Pd-膦预催化剂进行Suzuki-Miyaura酰胺交叉偶联的通用方法。最值得注意的是,该方法代表了在新兴的酰胺键交叉偶联歧管中使用实用且操作简单的Pd(II)-膦预催化剂的第一个例子。该反应在0.10摩尔%的负载下有效,从而提供了对N-C(O)键裂解具有高化学选择性的联芳基酮。这种通用的方法首次实现了在ppm负载下Pd-膦催化的酰胺交叉偶联。这种C-N交叉偶联可用于通过正交的Pd催化交叉偶联有效地提供药物中间体。我们完全希望操作简单[[PR 3)2 Pd(II)X 2]催化剂作为NC(O)交叉偶联的有效诱因将具有广泛的合成和催化价值。
更新日期:2020-04-03
中文翻译:

Suzuki-Miyaura使用良好定义的,稳定的[[PR3)2Pd(II)X2]预催化剂对酰胺进行交叉偶联
报道了一种使用高活性,定义明确且空气稳定的Pd-膦预催化剂进行Suzuki-Miyaura酰胺交叉偶联的通用方法。最值得注意的是,该方法代表了在新兴的酰胺键交叉偶联歧管中使用实用且操作简单的Pd(II)-膦预催化剂的第一个例子。该反应在0.10摩尔%的负载下有效,从而提供了对N-C(O)键裂解具有高化学选择性的联芳基酮。这种通用的方法首次实现了在ppm负载下Pd-膦催化的酰胺交叉偶联。这种C-N交叉偶联可用于通过正交的Pd催化交叉偶联有效地提供药物中间体。我们完全希望操作简单[[PR 3)2 Pd(II)X 2]催化剂作为NC(O)交叉偶联的有效诱因将具有广泛的合成和催化价值。










































 京公网安备 11010802027423号
京公网安备 11010802027423号