当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyclo-oligomerization of hydroxyl-containing mono-functional benzoxazines: a mechanism for oligomer formation
Polymer Chemistry ( IF 4.1 ) Pub Date : 2020/03/11 , DOI: 10.1039/d0py00047g Hecheng Zhen 1, 2, 3, 4, 5 , Huiyun Yang 1, 2, 3, 4, 5 , Mei Wang 1, 2, 3, 4, 5 , Guosheng Lu 1, 2, 3, 4, 5 , Yanfang Liu 1, 2, 3, 4, 5 , Mingtao Run 1, 2, 3, 4, 5
Polymer Chemistry ( IF 4.1 ) Pub Date : 2020/03/11 , DOI: 10.1039/d0py00047g Hecheng Zhen 1, 2, 3, 4, 5 , Huiyun Yang 1, 2, 3, 4, 5 , Mei Wang 1, 2, 3, 4, 5 , Guosheng Lu 1, 2, 3, 4, 5 , Yanfang Liu 1, 2, 3, 4, 5 , Mingtao Run 1, 2, 3, 4, 5
Affiliation
|
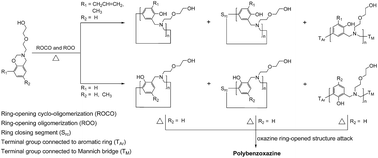
The oligomerization of mono-functional benzoxazines is demonstrated with intermolecular cyclization via a Mannich bridge structure as the primary reaction pathway. The structures of oligomers of mono-functional benzoxazines were previously thought to be benzoxazine dimers stabilized by inter- and intramolecular hydrogen bonding. This study is based on four hydroxyl-containing mono-functional benzoxazines synthesized from phenol, o-allylphenol, o-cresol, p-cresol, diglycolamine, and paraformaldehyde. A reaction mechanism is proposed that ortho- and para-substituted-phenol-based mono-functional benzoxazines undergo thermally activated ring-opening cyclo-oligomerization (ROCO) and ring-opening oligomerization (ROO), whereas their phenol-based counterpart polymerizes into polybenzoxazine following ROCO and ROO, which is evidenced by the data of 1H and 13C nuclear magnetic resonance spectra, Fourier transform infrared spectra, size exclusion chromatograms, and mass spectra of thermally treated samples.
中文翻译:

含羟基的单官能苯并恶嗪的环低聚:低聚物形成的机制
单功能苯并恶嗪的低聚反应通过曼尼希桥结构作为主要反应途径,通过分子间环化得到证明。以前认为单官能苯并恶嗪的低聚物的结构是通过分子间和分子内氢键稳定的苯并恶嗪二聚体。本研究是基于从苯酚合成了4种含羟基的单官能苯并恶嗪,ö -allylphenol,ø甲酚,p甲酚,二甘醇胺,和多聚甲醛。提出了邻位和对位的反应机理取代酚基单官能苯并恶嗪经历热活化的开环低聚(ROCO)和开环低聚(ROO),而它们的酚基对应物在ROCO和ROO之后聚合成聚苯并恶嗪,这由的数据1 H和13 C核磁共振光谱,傅里叶变换红外光谱,尺寸排阻色谱,并进行热处理的样品的质谱。
更新日期:2020-03-31
中文翻译:

含羟基的单官能苯并恶嗪的环低聚:低聚物形成的机制
单功能苯并恶嗪的低聚反应通过曼尼希桥结构作为主要反应途径,通过分子间环化得到证明。以前认为单官能苯并恶嗪的低聚物的结构是通过分子间和分子内氢键稳定的苯并恶嗪二聚体。本研究是基于从苯酚合成了4种含羟基的单官能苯并恶嗪,ö -allylphenol,ø甲酚,p甲酚,二甘醇胺,和多聚甲醛。提出了邻位和对位的反应机理取代酚基单官能苯并恶嗪经历热活化的开环低聚(ROCO)和开环低聚(ROO),而它们的酚基对应物在ROCO和ROO之后聚合成聚苯并恶嗪,这由的数据1 H和13 C核磁共振光谱,傅里叶变换红外光谱,尺寸排阻色谱,并进行热处理的样品的质谱。











































 京公网安备 11010802027423号
京公网安备 11010802027423号