Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH-Dependent transfer hydrogenation or dihydrogen release catalyzed by a [(η6-arene)RuCl(κ2-N,N-dmobpy)]+ complex: a DFT mechanistic understanding
RSC Advances ( IF 3.9 ) Pub Date : 2020-3-11 , DOI: 10.1039/c9ra10651k Chenguang Luo 1 , Longfei Li 2 , Xin Yue 1 , Pengjie Li 1 , Lin Zhang 1 , Zuoyin Yang 1 , Min Pu 1 , Zexing Cao 3 , Ming Lei 1, 3
RSC Advances ( IF 3.9 ) Pub Date : 2020-3-11 , DOI: 10.1039/c9ra10651k Chenguang Luo 1 , Longfei Li 2 , Xin Yue 1 , Pengjie Li 1 , Lin Zhang 1 , Zuoyin Yang 1 , Min Pu 1 , Zexing Cao 3 , Ming Lei 1, 3
Affiliation
|
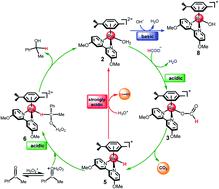
The reaction mechanism of the pH-dependent transfer hydrogenation of a ketone or the dehydrogenation of formic acid catalyzed by a [(η6-arene)RuCl(κ2-N,N-dmobpy)]+ complex in aqueous media has been investigated using the density functional theory (DFT) method. The TM-catalyzed TH of ketones with formic acid as the hydrogen source proceeds via two steps: the formation of a metal hydride and the transfer of the hydride to the substrate ketone. The calculated results show that ruthenium hydride formation is the rate-determining step. This proceeds via an ion-pair mechanism with an energy barrier of 14.1 kcal mol−1. Interestingly, the dihydrogen release process of formic acid and the hydride transfer process that produces alcohols are competitive under different pH environments. The investigation explores the feasibility of the two pathways under different pH environments. Under acidic conditions (pH = 4), the free energy barrier of the dihydrogen release pathway is 4.5 kcal mol−1 that is higher than that of the hydride transfer pathway, suggesting that the hydride transfer pathway is more favorable than the dihydrogen release pathway. However, under strongly acidic conditions, the dihydrogen release pathway is more favorable compared to the hydride transfer pathway. In addition, the ruthenium hydride formation pathway is less favorable than the ruthenium hydroxo complex formation pathway under basic conditions.
中文翻译:

[(η6-芳烃)RuCl(κ2-N,N-dmobpy)]+复合物催化的pH依赖性转移氢化或二氢释放:DFT机制理解
使用 [(η 6 -芳烃)RuCl(κ 2 - N , N -dmobpy)] +配合物在水介质中催化 pH 依赖性酮转移氢化或甲酸脱氢的反应机理密度泛函理论(DFT)方法。以甲酸为氢源的 TM 催化酮的 TH通过两个步骤进行:金属氢化物的形成以及氢化物转移到底物酮。计算结果表明氢化钌的形成是决定速率的步骤。这是通过能量垒为 14.1 kcal mol -1的离子对机制进行的。有趣的是,甲酸的氢气释放过程和产生醇的氢化物转移过程在不同pH环境下具有竞争性。本研究探讨了两种途径在不同pH环境下的可行性。在酸性条件下(pH = 4),氢气释放途径的自由能垒为4.5 kcal mol -1 ,高于氢化物转移途径的自由能垒,表明氢化物转移途径比氢气释放途径更有利。然而,在强酸性条件下,与氢化物转移途径相比,氢气释放途径更有利。此外,在碱性条件下,氢化钌形成途径不如钌羟基络合物形成途径有利。
更新日期:2020-03-11
中文翻译:

[(η6-芳烃)RuCl(κ2-N,N-dmobpy)]+复合物催化的pH依赖性转移氢化或二氢释放:DFT机制理解
使用 [(η 6 -芳烃)RuCl(κ 2 - N , N -dmobpy)] +配合物在水介质中催化 pH 依赖性酮转移氢化或甲酸脱氢的反应机理密度泛函理论(DFT)方法。以甲酸为氢源的 TM 催化酮的 TH通过两个步骤进行:金属氢化物的形成以及氢化物转移到底物酮。计算结果表明氢化钌的形成是决定速率的步骤。这是通过能量垒为 14.1 kcal mol -1的离子对机制进行的。有趣的是,甲酸的氢气释放过程和产生醇的氢化物转移过程在不同pH环境下具有竞争性。本研究探讨了两种途径在不同pH环境下的可行性。在酸性条件下(pH = 4),氢气释放途径的自由能垒为4.5 kcal mol -1 ,高于氢化物转移途径的自由能垒,表明氢化物转移途径比氢气释放途径更有利。然而,在强酸性条件下,与氢化物转移途径相比,氢气释放途径更有利。此外,在碱性条件下,氢化钌形成途径不如钌羟基络合物形成途径有利。









































 京公网安备 11010802027423号
京公网安备 11010802027423号