The Lancet Respiratory Medicine ( IF 38.7 ) Pub Date : 2020-03-11 , DOI: 10.1016/s2213-2600(20)30053-9 Ian D Pavord 1 , Mark Holliday 2 , Helen K Reddel 3 , Irene Braithwaite 2 , Stefan Ebmeier 2 , Robert J Hancox 4 , Tim Harrison 5 , Claire Houghton 2 , Karen Oldfield 2 , Alberto Papi 6 , Mathew Williams 2 , Mark Weatherall 7 , Richard Beasley 8 ,
|
Background
Whether blood eosinophil counts and exhaled nitric oxide (FeNO) are associated with important outcomes in mild asthma is unclear. In this prespecified subgroup analysis of a previously published open-label clinical trial, we aimed to assess associations between blood eosinophil counts and FeNO with outcomes and response to asthma treatment.
Methods
In the previously reported 52-week, open-label, randomised controlled trial, people with mild asthma receiving only β agonist reliever inhalers were enrolled at one of 16 clinical trials units in New Zealand, the UK, Italy, or Australia. Eligible participants were randomly assigned (1:1:1, stratified by country), to receive inhalers to take as-needed salbutamol (two inhalations of 100 μg in a pressurised metered dose inhaler), maintenance budesonide (200 μg twice per day by inhaler) plus as-needed salbutamol (two inhalations of 100 μg), or as-needed budesonide–formoterol (one inhalation of 200 μg budesonide and 6μg formoterol by inhaler). The primary outcome was the annual rates of asthma exacerbations per patient, and in this prespecified subgroup analysis, we assessed whether annual exacerbation rates in each treatment group were significantly different depending on levels of blood eosinophil count, FeNO, or a composite score of both. Analyses were done for patients with available biomarker measurements The study was registered with the Australian New Zealand Clinical Trials Registry, number ACTRN12615000999538.
Findings
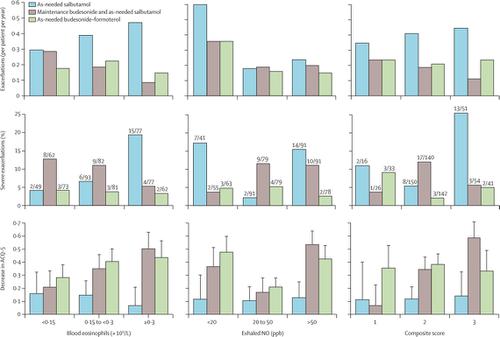
675 participants were enrolled between March 17, 2016, and Aug 29, 2017, of whom 656 had results for blood eosinophil analysis and 668 had results for FeNO. Of the patients who received as-needed salbutamol, the proportion of patients having a severe exacerbation increased progressively with increasing blood eosinophil count (two [4%] of 49 participants with <0·15 × 109/L, six [6%] of 93 with 0·15 to <0·3 × 109/L, and 15 [19%] of 77 with ≥0·3 × 109/L; p=0·014). There were no significant interactions between blood eosinophil count or FeNO level and the effect of as-needed budesonide–formoterol compared with as-needed salbutamol for either exacerbations or severe exacerbations. However, there were significant interactions between blood eosinophil count subgroups and the effect of maintenance budesonide plus as-needed salbutamol compared with as-needed salbutamol, both for exacerbations (p=0·0006) and severe exacerbations (p=0·0007). Maintenance budesonide plus as-needed salbutamol was more effective than as-needed salbutamol in patients with blood eosinophil counts of 0·3 × 109/L or more, both for exacerbations (rate ratio 0·13 [95% CI 0·05–0·33]) and severe exacerbations (risk odds ratio 0·11 [0·03–0·45]). This difference was not seen for blood eosinophil counts of less than 0·15 × 109/L (1·15 [0·51–1·28] for exacerbations and 5·72 [0·97–33·60] for severe exacerbations). There was no consistent interaction between treatment response and FeNO or the composite score.
Interpretation
In patients with mild asthma, the effects of as-needed budesonide–formoterol on exacerbations are independent of biomarker profile, whereas the benefits of maintenance inhaled budesonide are greater in patients with high blood eosinophil counts than in patients with low counts.
Funding
AstraZeneca, Health Research Council of New Zealand.
中文翻译:

血液嗜酸性粒细胞和呼出气一氧化氮对轻度哮喘成年人的预测价值:一项开放标签,平行分组,随机对照试验的预先确定的亚组分析。
背景
尚不清楚血液嗜酸性粒细胞计数和呼出气一氧化氮(FeNO)是否与轻度哮喘的重要预后相关。在先前公开的开放标签临床试验的这一预先指定的亚组分析中,我们旨在评估血液嗜酸性粒细胞计数和FeNO与结局和对哮喘治疗的反应之间的关联。
方法
在先前报道的一项为期52周的开放标签,随机对照试验中,仅在新西兰,英国,意大利或澳大利亚的16个临床试验单位之一中入选了仅接受β激动剂缓解剂吸入的轻度哮喘患者。合格的参与者被随机分配(按国家划分为1:1:1),接受吸入剂以按需服用沙丁胺醇(在加压计量吸入器中两次吸入100μg),维持布地奈德(每天200μg,吸入器两次) )加上需要的沙丁胺醇(两次吸入100μg),或需要的布地奈德-福莫特罗(一次吸入200μg布地奈德和6μg福莫特罗)。主要结局是每位患者的哮喘急性发作率,在此预先设定的亚组分析中,我们评估了每个治疗组的年急性加重率是否显着不同,这取决于血液嗜酸性粒细胞计数,FeNO或两者的综合评分。对具有可用生物标志物测量值的患者进行了分析。该研究已在澳大利亚新西兰临床试验注册中心注册,编号为ACTRN12615000999538。
发现
在2016年3月17日至2017年8月29日之间招募了675名参与者,其中656名血液嗜酸性粒细胞分析结果和668名FeNO结果。接受急需沙丁胺醇的患者中,严重加重患者的比例随着血液嗜酸性粒细胞计数的增加而逐渐增加(49名参与者中,<0·15×10 9 / L的参与者中有2名[4%] ,有6名[6%] 93(0·15至<0·3×10 9 / L)和15 [19%](77)中≥0·3×10 9/ L; p = 0·014)。急性加重或严重加重时,血液嗜酸性粒细胞计数或FeNO水平与需要的布地奈德-福莫特罗与需要的沙丁胺醇之间没有明显的相互作用。然而,无论是急性加重(p = 0·0006)还是重度加重(p = 0·0007),血液嗜酸性粒细胞计数亚组与维持布地奈德加急需的沙丁胺醇与急需的沙丁胺醇的作用均存在显着的相互作用。在血液嗜酸性粒细胞计数为0·3×10 9的患者中,维持布地奈德加需要的沙丁胺醇比需要的沙丁胺醇更有效/ L或更高,对于加重(比率比率0·13 [95%CI 0·05-0·33])和严重加重(风险几率比率0·11 [0·03-0·45])都是如此。对于血液嗜酸性粒细胞计数低于0·15×10 9 / L(加重为1·15 [0·51–1·28],严重程度为5·72 [0·97–33·60],则看不到这种差异)恶化)。治疗反应与FeNO或复合评分之间没有一致的相互作用。
解释
在轻度哮喘患者中,布地奈德-福莫特罗按需加重的影响与生物标志物的分布无关,而血液嗜酸性粒细胞计数高的患者比低计数的患者维持吸入布地奈德的益处更大。
资金
阿斯利康,新西兰卫生研究委员会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号